Another important aspect of IS impact on their bacterial hosts is their ability to modulate gene expression. In addition to acting as vectors for gene transmission from one replicon to another in the form composite transposons (two IS flanking any gene; Fig.2.3) and tIS (Fig.8.1) and their ability to interrupt genes, it has been known for some time[1][2] that IS can also activate gene expression. This capacity has recently received much attention due to the increase in resistance to various antibacterials[3][4][5], a worrying public health threat[6][7].
They can accomplish this in two ways: either by providing internal promoters whose transcripts escape into neighboring DNA[2][8][9][10] or by hybrid promoter formation. Many IS carry -35 promoter components oriented towards the flanking DNA (Fig.18.1). In a number of cases this plays an important part in their transposition since a significant number of IS transposes using an excised transposon circle (Fig.18.1) with abutted left and right ends. For these IS, the other end carries a -10 element oriented inwards towards the Tpase gene. Together with the -35, this generates a strong promoter on formation of the circle junction to drive Tpase expression required for catalysis of integration (Fig.18.2) [11][12][13][14]. Thus, if integration occurs next to a resident -10 sequence, the IS -35 sequence can contribute to a hybrid promoter to drive expression of neighboring genes [see [15]]. At present, this phenomenon had been reported to occur with over 30 different IS in more than 17 bacterial species[16][17] (Table IS and Gene Expression below). Indeed, specific vector plasmids have been designed to identify activating insertions (e.g. [18]).
IS activity can affect efflux mechanisms resulting in increased resistance: IS1 or IS10 insertion can up-regulate the AcrAB-TolC pump in Salmonella enterica[19]; IS1 or IS2 insertion upstream of AcrEF[20][21] and IS186 insertional inactivation of the AcrAB repressor, AcrR, in Escherichia coli [20], all lead to increased resistance to fluoroquinolones. Insertional inactivation of specific porins can also play a significant role[22].
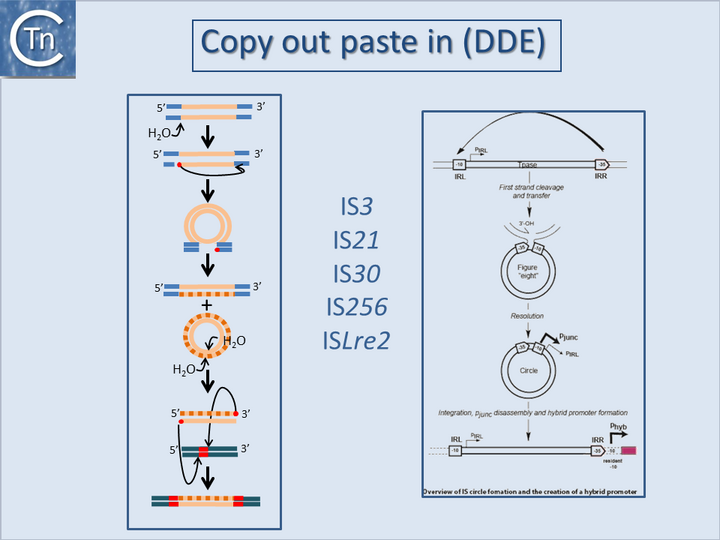
Fig.18.1. Copy out, paste in (DDE).
Left column. Illustrates the copy out paste in the transposition mechanism. The transposon is represented as a yellow line. Flanking sequences in the donor molecule are blue. Flanking sequences in the target molecule are green. Red circles indicate 3′OH moieties generated by Tpase-catalyzed hydrolysis at the transposon end(s). Red boxes indicate target DNA flanks that are duplicated on insertion. Top to bottom: Tpase catalyzed cleavage at the 3′ transposon ends using H2O as the nucleophile. Liberated 3′OH attack the opposite end to create a bridged molecule which then undergoes replication (dotted line) to generate a circular transponso copy and regenerate the donor molecule. The circle intermediate undergoes cleavage to generate two 3'OH ends which attack the target DNA in a staggered way, resulting in integration. Repair at each end then generates the direct target repeat characteristic of many transposons.
Right column. Formation of hybrid promoters by copy out -paste in transposition. The left and right terminal inverted repeats of the IS, IRL ,and IRR, are shown as a square and pointed box respectively. The component –10 and –35 promoter elements of p
junc within these ends are also shown, together with the weak p
IRL promoter and the direction of transcription of the transposase. In a first step, single-strand cleavage and transfer from one IR to the other is catalyzed by OrfAB produced from the weak p
IRL promoter. This is indicated at the top of the figure by the curved arrow. In this case, the right end (IRR) is shown attacking the left end (IRL). The resulting figure-eight form is drawn below and shows the free 3’OH group generated on the flanking donor DNA sequence. In a second step, second-strand circularization occurs by an as yet undetermined mechanism involving host functions but independently of transposon proteins [Turlan et al., 2000]. The resulting IRR-IRL junction carries suitably placed -35 and –10 hexamers, separated by a canonical 17 bp spacer and form a strong p
junc (bold arrow) promoter able to promote high levels of production of IS
911 proteins. Integration of the circle results in disassembly of the promoter restoring low levels of expression from p
IRL. Insertion upstream of a resident -10 promoter element can bring the IR-associated -35 element at the correct distance to form a promoter and activate a downstream gene.
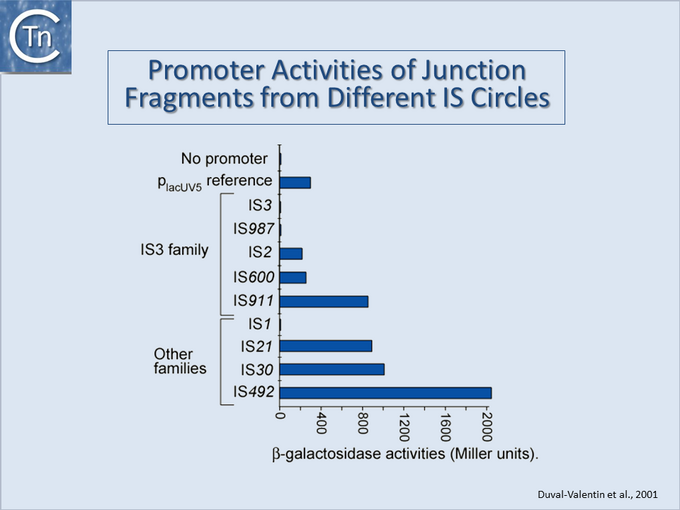
Fig.18.2. Promoter Activities of junction fragments from different IS circles. Results of measurements of activities of 9 IS circle junctions when placed upstream of a
beta-galactosidase gene.
IS and Gene Expression
| Table. IS and gene expression.
|
| IS family |
IS name |
Mechanism |
Gene(s) affected |
Organism |
Reference |
Clinical/Experimental
|
| IS1 |
IS1 |
Cointegrate |
EmR |
Escherichia coli |
[23] |
C?
|
| Copy-paste circles |
blaTEM-1 |
[15] |
E
|
| — |
acrEF pump |
Salmonella enterica |
[19] |
E
|
| IS3 |
IS2 |
Copy-paste circles |
gal |
Escherichia coli |
[24][25] |
E
|
| gal |
[23][2] |
E
|
| argE |
[26][20] |
E
|
| EmR |
C?
|
| blaampC |
E
|
| acrEF pump |
E
|
| IS3 |
Copy-paste circles |
argE |
Escherichia coli |
[2] |
E
|
| argE |
[8] |
E
|
| citT |
[27] |
E
|
| IS981 |
Copy-paste circles |
ldhB |
Lactococcus lactis |
[28] |
E
|
| IS6110 |
Copy-paste circles |
Rv2280 and PE-PGRS gene, Rv1468c |
Mycobacterium tuberculosis |
[29] |
E
|
| ISKpn8 |
Copy-paste circles |
blaKCP-2 |
Escherichia coli, Citrobacter freundii, Enterobacter cloacae, Enterobacter aerogenes, and Klebsiella oxytoca |
[30] |
C
|
| IS4 |
IS10 |
Cut-paste (hairpin) |
his |
Salmonella typhimurium |
[31] |
E
|
| Cut-paste |
— |
Escherichia coli |
[32] |
E
|
| Cut-paste |
acrEF pump |
Salmonella enterica |
[19] |
E
|
| IS50 |
Cut-paste (hairpin) |
aph3’II |
Escherichia coli |
[33] |
E
|
| IS1999 |
— |
blaVEB-1 |
Pseudomonas aeruginosa |
[4] |
C
|
| blaVEB-1/blaOXA-48 |
Escherichia coli |
[3] |
C
|
| ISPa12 |
— |
blaPER-1 |
Salmonella enterica, Pseudomonas aeruginosa, Providencia stuartii, Acinetobacter baumannii |
[34] |
C
|
| ISAba1 |
— |
blaampC |
Acinetobacter baumannii |
[35][36][37] |
C
|
| blaOXA-51/blaOXA-23 |
[38] |
C
|
| IS5
|
IS5 |
— |
EmR |
Escherichia coli |
[23] |
C?
|
| ISFtu2 |
general |
Francisella tularensis |
[39] |
Natural isolate
|
| ISVa1 |
(iron uptake) |
Vibrio anguilarum |
[40] |
Natural isolate
|
| IS1168 |
nimA, nimB |
Bacteroides sp. |
[41] |
C
|
| IS1186 |
cfiA |
Bacteroides fragilis |
[42][43] |
C
|
| IS402 |
bla |
Pseudomonas cepacia |
[44] |
E
|
| IS6
|
IS257 |
Cointegrate |
dfrA |
Staphylococcus aureus |
[45] |
C
|
| IS257 |
Cointegrate |
tet |
Staphylococcus aureus |
[46]
|
| IS1008 |
— |
blaOXA-58 |
Acinetobacter baumannii |
[47]
|
| IS26 |
— |
aphA7, blaS2A |
Klebsiella pneumoniae |
[48]
|
| blaSHV-2a |
Pseudomonas aeruginosa |
[49]
|
| IS140 |
— |
aac(3)-III and -IV |
— |
[50]
|
| IS21
|
ISBf1 |
Copy-paste circle |
cepA |
Bacteroides fragilis |
[51] |
C
|
| ISKpn7 |
blaKPC |
Klebsiellea pneumonia |
[52]
|
| IS30
|
IS30 |
Copy-paste circle |
galK |
Escherichia coli |
[53] |
E
|
| IS18 |
aac(6’)-Ij |
Acinetobacter sp. |
[54] |
C
|
| blaOXA257 |
[55]
|
| IS4351 |
ermF |
Bacteroides fragilis |
[56] |
C
|
| cfiA |
[5]
|
| IS1086 |
cnrCBAT (ZnR) |
Cupriavidus metallidurans |
|
E
|
| IS256
|
IS256 |
Copy-paste circles |
mecA |
Staphylococcus sciuri |
[57][58] |
—
|
| llm |
Staphylococcus aureus
|
| IS1490 |
— |
Burkholderia cepacia |
[59] |
—
|
| IS406 |
— |
Burkholderia cepacia |
[44] |
E
|
| IS481 |
IS481 |
Copy-paste circles |
katA |
Bordetella pertussis |
[60] |
—
|
| ISRme5 |
cnrCBAT (Zn R) |
Cupriavidus metallidurans |
[61] |
E
|
| IS630 |
ISFtu1 |
Tc-like |
general |
Francisella tularensis |
[39] |
Natural isolate
|
| IS982
|
IS1187 |
— |
cfiA |
Bacteroides fragilis |
[42] |
E+C
|
| [5][62][63] |
C
|
| IS982 |
citQRP |
Lactococcus lactis |
[64] |
—
|
| IS1380
|
ISBf12 |
— |
cfiA |
Bacteroides fragilis |
[5] |
C
|
| IS612 |
[63]
|
| IS613 |
[63]
|
| IS614 |
[5][63][65]
|
| IS1188 |
[62]
|
| IS942 |
[62]
|
| ISEcp1 |
blaCTX-M-15 |
Enterobacteriaceae |
[66]
|
| blaCTX-M-17 |
Klebsiella pneumoniae |
[67]
|
| blaCTX-M-19 |
Klebsiella pneumoniae |
[34]
|
| blaCTX-M |
Kluyvera ascorbata |
[68]
|
| rmtC |
Escherichia coli |
[69]
|
| IS1187 |
cfiA |
Bacteroides fragilis |
[62]
|
| ISL3
|
ISSg1 |
Copy-paste circles |
sspB (surface antigen) |
Streptococcus gordonii |
[70] |
—
|
| IS1411 |
pheBA |
Pseudomonas putida |
[71]
|
| ISAs1
|
IS1548 |
— |
lmb (lamelin binding) |
Streptococcus agalactiae |
[72] |
—
|
| nd |
— |
Acinetobacter baumannii |
[35] |
C
|
| ISNYC
|
IS403 |
— |
bla |
Burkholderia cepacia |
[44] |
E
|
| IS404
|
| IS405
|
Bibliography
- ↑ <pubmed>1101028</pubmed>
- ↑ 2.0 2.1 2.2 2.3
</nowiki>
- ↑ 3.0 3.1
Aubert D, Naas T, Héritier C, Poirel L, Nordmann P . Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. - J Bacteriol: 2006 Sep, 188(18);6506-14 [PubMed:16952941]
[DOI]
</nowiki>
- ↑ 4.0 4.1
</nowiki>
- ↑ 5.0 5.1 5.2 5.3 5.4
Sóki J, Eitel Z, Urbán E, Nagy E, ESCMID Study Group on Anaerobic Infections . Molecular analysis of the carbapenem and metronidazole resistance mechanisms of Bacteroides strains reported in a Europe-wide antibiotic resistance survey. - Int J Antimicrob Agents: 2013 Feb, 41(2);122-5 [PubMed:23158541]
[DOI]
</nowiki>
- ↑ <pubmed>23887414</pubmed>
- ↑ <pubmed>23887415</pubmed>
- ↑ 8.0 8.1
</nowiki>
- ↑ <pubmed>6260746</pubmed>
- ↑ <pubmed>6311437</pubmed>
- ↑ <pubmed>26350305</pubmed>
- ↑ <pubmed>9214651</pubmed>
- ↑ <pubmed>10438765</pubmed>
- ↑ <pubmed>11598022</pubmed>
- ↑ 15.0 15.1
Prentki P, Teter B, Chandler M, Galas DJ . Functional promoters created by the insertion of transposable element IS1. - J Mol Biol: 1986 Oct 5, 191(3);383-93 [PubMed:3029382]
[DOI]
</nowiki>
- ↑ <pubmed>17223624</pubmed>
- ↑ <pubmed>24499397</pubmed>
- ↑ <pubmed>8863735</pubmed>
- ↑ 19.0 19.1 19.2
</nowiki>
- ↑ 20.0 20.1 20.2
Jellen-Ritter AS, Kern WV . Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants Selected with a fluoroquinolone. - Antimicrob Agents Chemother: 2001 May, 45(5);1467-72 [PubMed:11302812]
[DOI]
</nowiki>
- ↑ <pubmed>11274125</pubmed>
- ↑ <pubmed>15212803</pubmed>
- ↑ 23.0 23.1 23.2
</nowiki>
- ↑ <pubmed>4610339</pubmed>
- ↑ <pubmed>339095</pubmed>
- ↑ <pubmed>6187472</pubmed>
- ↑ <pubmed>22992527</pubmed>
- ↑ <pubmed>12867459</pubmed>
- ↑ <pubmed>15130120</pubmed>
- ↑ <pubmed>24433026</pubmed>
- ↑ <pubmed>6289329</pubmed>
- ↑ <pubmed>6311437</pubmed>
- ↑ <pubmed>6260374</pubmed>
- ↑ 34.0 34.1
</nowiki>
- ↑ 35.0 35.1
</nowiki>
- ↑ <pubmed>14742218</pubmed>
- ↑ <pubmed>16441449</pubmed>
- ↑ <pubmed>16630258</pubmed>
- ↑ 39.0 39.1
</nowiki>
- ↑ <pubmed>7568465</pubmed>
- ↑ <pubmed>8067736</pubmed>
- ↑ 42.0 42.1
</nowiki>
- ↑ <pubmed>7545155</pubmed>
- ↑ 44.0 44.1 44.2
Scordilis GE, Ree H, Lessie TG . Identification of transposable elements which activate gene expression in Pseudomonas cepacia. - J Bacteriol: 1987 Jan, 169(1);8-13 [PubMed:3025189]
[DOI]
</nowiki>
- ↑ <pubmed>7840551</pubmed>
- ↑ <pubmed>10852863</pubmed>
- ↑ <pubmed>18443121</pubmed>
- ↑ <pubmed>2160941</pubmed>
- ↑ <pubmed>10223953</pubmed>
- ↑ <pubmed>6318050</pubmed>
- ↑ <pubmed>7517394</pubmed>
- ↑ <pubmed>18227185</pubmed>
- ↑ <pubmed>3039299</pubmed>
- ↑ <pubmed>9756793</pubmed>
- ↑ <pubmed>23014718</pubmed>
- ↑ <pubmed>3038844</pubmed>
- ↑ <pubmed>9371438</pubmed>
- ↑ <pubmed>12511511</pubmed>
- ↑ <pubmed>9098071</pubmed>
- ↑ <pubmed>7830550</pubmed>
- ↑ <pubmed>27047473</pubmed>
- ↑ 62.0 62.1 62.2 62.3
</nowiki>
- ↑ 63.0 63.1 63.2 63.3
</nowiki>
- ↑ <pubmed>8602160</pubmed>
- ↑ <pubmed>19744834</pubmed>
- ↑ <pubmed>11470367</pubmed>
- ↑ <pubmed>12435670</pubmed>
- ↑ <pubmed>16569841</pubmed>
- ↑ <pubmed>16940134</pubmed>
- ↑ <pubmed>9202480</pubmed>
- ↑ <pubmed>9765560</pubmed>
- ↑ <pubmed>20520730</pubmed>

