IS Families/IS200-IS605 family
Contents
- 1 Historical
- 2 General
- 3 Distribution and Organization
- 4 Mechanism of IS200/IS605 single strand DNA transposition
- 5 Regulation of single strand transposition
- 6 TnpB and its Relatives: Guide RNA Endonucleases
- 7 ncRNAs, sotRNAs and reRNAs
- 8 RNA Nomenclature, Processing, Structure, Diversity and mode of function
- 9 The IS1341 Conundrum: how do derivatives without their transposase transpose?
- 9.1 IS1341 Group Diversity: Mining the NCBI NR database
- 9.2 Conserved secondary structure motifs
- 9.3 IS1341 group orientation suggests iscB re(ω)RNA but not tnpB re(ω)RNA is expressed in transcriptionally active environments.
- 9.4 IS1341 Group Function
- 9.5 Does a Resident TnpA copy Drive IS1341 group Transposition?
- 9.6 TnpBGst and IscBGst proteins are active RNA-guided Nucleases
- 9.7 TnpB is Required for Replacement of the Deleted IS Copy
- 9.8 The Copy Choice Model for TnpB Function During Transposition
- 10 IStrons
- 10.1 The IS605-based IStron: CdiIStron
- 10.2 TnpAS IS607 Excision and Insertion Activity
- 10.3 IStron-encoded TnpB nucleases
- 10.4 Defining the CboIStron TAM Sequence: a double role in both nuclease and transposase recognition
- 10.5 CboIStron TnpB/wRNA promotes transposon copy number maintenance
- 10.6 Busy Ends: Functional interactions between IStron splicing, TnpB and ωRNA
- 11 The Eukaryotic Connection: Fanzor eukaryotic TnpB relatives
- 12 Y1 transposase domestication
- 13 IS200 Regulation and Salmonella Pathogenicity
- 13.1 Transposase expression is regulated by an antisense RNA
- 13.2 Transposase expression is regulated by an antisense RNA and Hfq
- 13.3 What is the impact of IS200 on its host genome?
- 13.4 Which host genes might be regulated by IS200 RNA?
- 13.5 5’UTR RNA is processed.
- 13.6 The processed RNA represses SPI-1 genes by repressing invF transcriptional activator transcription.
- 13.7 Direct tnpA RNA-invF RNA Interaction in vivo and in vitro.
- 13.8 Growth phase dependence.
- 13.9 TnpA controls expression of more than 200 host genes
- 13.10 A model of the IS200 regulatory network
- 14 TnpB co-option as transcription factors.
- 15 Acknowledgements
- 16 Bibliography
Historical
One of the founding members of this group, IS200, was identified in Salmonella typhimurium [1] as a mutation in hisD (hisD984) which mapped as a point mutation but which did not revert and was polar on the downstream hisC gene (see [2]). S. typhimurium LT2 was found to contain six IS200 copies and the IS was unique to Salmonella [3]. Further studies [4] showed that the IS did not carry repeated sequences, either direct or inverted, at its ends, and that removal of 50 bp at the transposase proximal end (which includes a structure resembling a transcription terminator) removed the strong transcriptional block. IS200 elements from S. typhimurium and S. abortusovis revealed a highly conserved structure of 707–708 bp with a single open-reading-frame potentially encoding a 151 aa peptide and a putative upstream ribosome-binding-site [5].
It has been suggested that a combination of inefficient transcription, protection from impinging transcription by a transcriptional terminator, and repression of translation by a stem-loop mRNA structure. All contribute to tight repression of transposase synthesis [2]. However, although IS200 seems to be relatively inactive in transposition [6], it is involved in chromosome arrangements in S. typhimurium by recombination between copies [7].
A second group of “founding” members of this family was, arguably, IS1341 from the thermophilic bacterium PS3 [8], IS891 from Anabaena sp. M-131 [9] and IS1136 from Saccharopolyspora erythraea [10]. The “transposases” of both elements were observed to be associated in a single IS, IS605, from the gastric pathogen Helicobacter pylori [11]. It was identified in many independent isolates of H. pylori and is now considered to be a central member which defines this large family. IS605 was shown to possess unique, not inverted repeat, ends; did not duplicate target sequences during transposition; and inserted with its left (IS200-homolog) end abutting 5'-TTTAA or 5'-TTTAAC target sequences [11]. Additionally, a second derivative, IS606, with only 25% amino acid identity in the two proteins (orfA and orfB) was also identified in many of the H. pylori isolates including some which were devoid of IS605. The Berg lab also identified another H. pylori IS, IS607 [12] which carried a similar IS1341-like orf (orfB) but with another upstream orf with similarities to that of the mycobacterial IS1535 [13] annotated as a resolvase due the presence of a site-specific serine recombinase motif. Another IS605 derivative, ISHp608, which appeared widely distributed in H. pylori was shown to transpose in E. coli, required only orfA to transpose and inserted downstream from a 5’-TTAC target sequence [14].
General
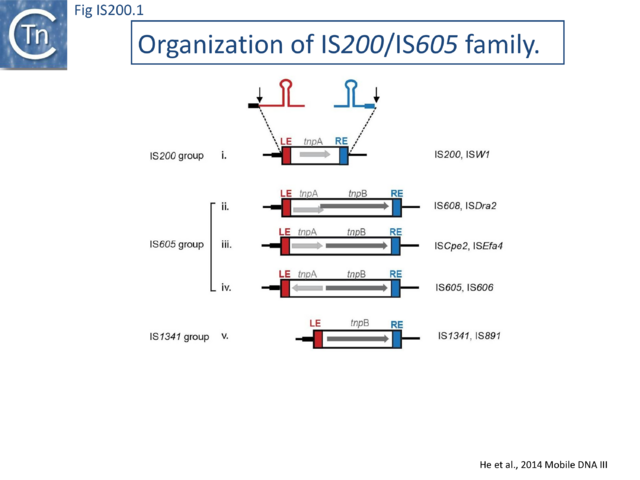
The IS200/IS605 family members transpose using obligatory single strand(ss) DNA intermediates [15] by a mechanism called “peel and paste”. They differ fundamentally in the organization from classical IS. They have sub-terminal palindromic structures rather than terminal IRs (Fig. IS200.1) and insert 3’ to specific AT-rich tetra- or penta-nucleotides without duplicating the target site.
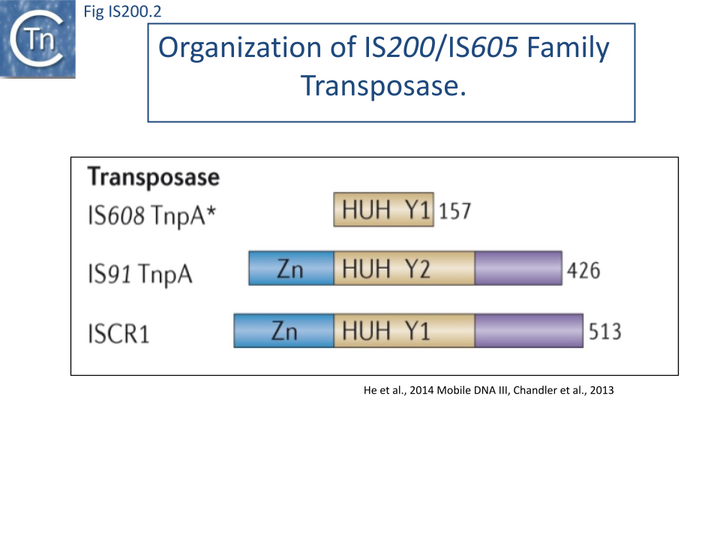
The transposase, TnpA, is a member of the HUH enzyme superfamily (Relaxases, Rep proteins of RCR plasmids/ss phages, bacterial and eukaryotic transposases of IS91/ISCR and Helitrons[16][17])(Fig. IS200.2) which all catalyze cleavage and rejoining of ssDNA substrates.
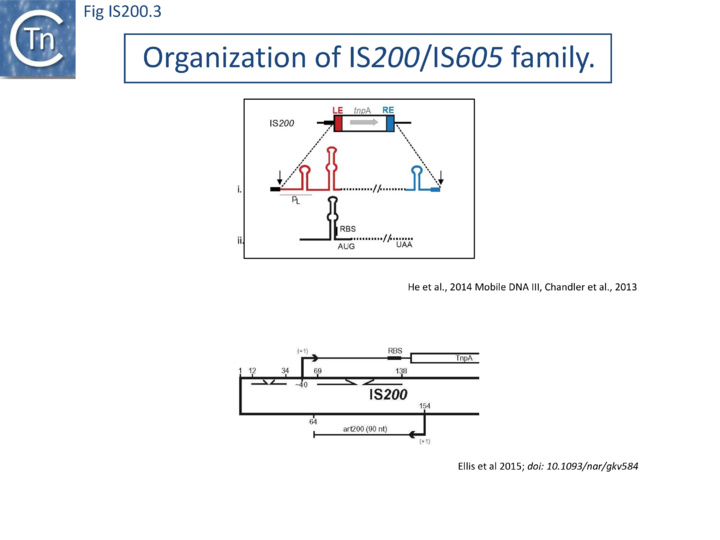
IS200, the founding member (Fig. IS200.3), was identified 30 years ago in Salmonella typhimurium [1] but there has been renewed interest for these elements since the identification of the IS605 group in Helicobacter pylori [11][18][14]. Studies of two elements of this group, IS608 from H. pylori and ISDra2 from the radiation resistant Deinococcus radiodurans, have provided a detailed picture of their mobility [19][20][21][22][23][24][25].
Distribution and Organization
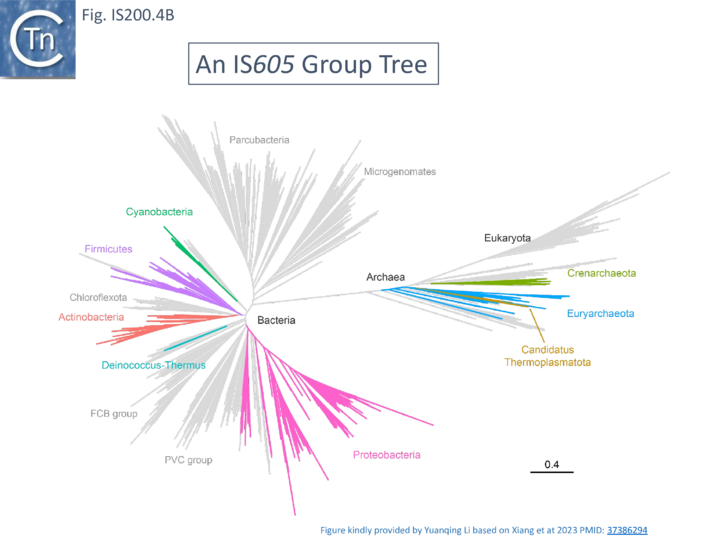
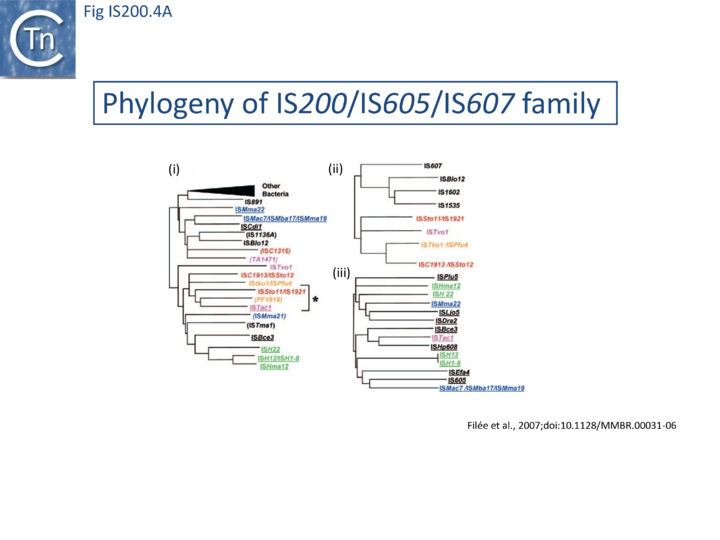
The family is widely distributed in prokaryotes with more than 153 distinct members (89 are distributed over 45 genera and 61 species of bacteria, and 64 are from archaea). It is divided into three major groups based on the presence or absence and on the configuration of two genes: the transposase tnpA (https://www.ncbi.nlm.nih.gov/research/cog/cog/COG1943/), sufficient to promote IS mobility in vivo and in vitro and tnpB (https://www.ncbi.nlm.nih.gov/research/cog/cog/COG0675/) (Fig. IS200.1) initially of unknown function and not required for transposition activity but now known to de an RNA-guide endonuclease (see TnpB below) . These groups are: IS200, IS605 and IS1341. TnpB is also present in another IS family, IS607, which uses a serine-recombinase as a transposase. In the phylogeny of this group (Fig. IS200.4A) of IS, both tnpB and tnpA of bacterial or archaeal origin are intercalated, suggesting some degree of horizontal transfer between these two groups of organisms[26].
Isolated copies of IS200-like tnpA can be identified in both bacteria and archaea[26]. Full length copies of IS605-like elements are also found in bacteria and several archaea and all have corresponding MITEs (Miniature Inverted repeat Transposable Elements) derivatives in their host genomes.
The IS200 group
IS200 group members encode only tnpA, and are present in gram-positive and gram-negative bacteria and certain archaea[2][27] (Fig. IS200.1 and Fig. IS200.3). Alignment of TnpA from various members shows that they are highly conserved but may carry short C-terminal tails of variable length and sequence. Among approximately 400 entries in ISfinder (December 2023), about 50 examples IS200-like derivatives.
They can occur in relatively high copy number (e.g. >50 copies of IS1541 in Yersinia pestis) and are among the smallest known autonomous IS with lengths generally between 600-700 pb. Some members such as ISW1 (from Wolbachia sp.) or ISPrp13 (from Photobacterium profundum) are even shorter.
IS200 was initially identified as an insertion mutation in the Salmonella typhimurium histidine operon [1]. It is abundant in different Salmonella strains and has now also been identified in a variety of other enterobacteria such as Escherichia, Shigella and Yersinia.
Different enterobacterial IS200 copies have almost identical lengths of between 707 and 711bp. Analysis of the ECOR (E. coli) and SARA (Salmonellae) collections showed that the level of sequence divergence between IS200 copies from these hosts is equivalent to that observed for chromosomally encoded genes from the same taxa[28][29]. This suggests that IS200 was present in the common ancestor of E. coli and Salmonellae.
In spite of their abundance, an enigma of IS200 behavior is its poor contribution to spontaneous mutation in its original Salmonella host: only very rare insertion events have been documented [2]. One reason for these rare insertions could be due to poor expression of the TnpAIS200 gene from a weak promoter pL identified at the left IS end (LE)[4][5] (Fig. IS200.3).
Besides the characteristic major subterminal palindromes [4] presumed binding sites of the transposase at both LE and the right end (RE) (Substrate recognition), IS200 carries also a potential supplementary interior stem-loop structure (Fig. IS200.3). These two structures play a role in regulating IS200 gene expression. The first (perfect palindrome at LE; nts 12–34) overlaps the TnpAIS200 promoter pL, can act as a bi-directional transcription terminator upstream of TnpAIS200 and terminates up to 80% of transcripts[30] (Fig. IS200.3). The second (interior stem-loop; nts 69–138) (Fig. IS200.3), at the RNA level, can repress mRNA translation by sequestration of the Ribosome Binding Site (RBS) (Fig. IS200.3). Experimental data suggested that the stem-loop is formed in vivo and its removal by mutagenesis caused up to a 10 fold increase in protein production[30]. Recent deep sequencing analysis revealed another aspect in post-transcriptional regulation of IS200 expression: A small anti-sense RNA (asRNA) IS200 transposase expression (Fig. IS200.3) was identified as a substrate of Hfq, an RNA chaperone involved in post-transcriptional regulation in numerous bacteria[31]. Interestingly, asRNA and Hfq independently inhibit IS200 transposase expression: knock-out of both components resulted in a synergistic increase in transposase expression. Moreover, footprint data showed that Hfq binds directly to the 5’ part of the transposase transcript and blocks access to the RBS[32].
In spite of its very low transposition activity, an increase in IS200 copy number was observed during strain storage in stab cultures[1][3]. However, the factors triggering this activity remain unknown[2] . Transient high transposase expression leading to a burst of transposition was proposed to explain the observed high IS200 (>20) copy number in various hosts and in stab cultures [1].
Although regulatory structures similar to that observed in IS200 (Fig. IS200.3) were predicted in IS1541, another member of this group with 85% identity to IS200, this element can be detected in higher copy number (> 50) in Salmonella and Yersinia genomes. However, no detailed analysis of its transposition is available and since no de novo insertions have been experimentally documented and chromosomal copies appear stable in Y. pestis[33], it remains possible that IS1541 also behaves like IS200.
However, the regulatory structures are not systematically present in other IS200 group members and understanding of the control of transposase synthesis requires further study.
The IS605 group
IS605 group members are generally longer (1.6-1.8 kb) due to the presence of a second orf, tnpB in addition to tnpA. Alignment of TnpA copies from this group indicated that although they do not form a separate clade from the IS200 group TnpA, they generally carry the short C-terminal tail. The tnpA and tnpB orfs exhibit various configurations with respect to each other. They may be divergent (Fig. IS200.1 i top: e.g. IS605, IS606) or expressed in the same direction with tnpA upstream of tnpB. In these latter cases, the orfs may be partially overlapping (Fig. IS200.1 ii; e.g. IS608, ISDra2) or separate Fig. IS200.1 iii; e.g. ISSCpe2, ISEfa4). tnpB is also sometimes associated with another transposase, a member of the S-transposases (e.g. IS607[12][34], see [15]. TnpB was not required for transposition of either IS608 or ISDra2.
Three related IS, IS605, IS606 and IS608 (Fig. IS200.1) have been identified in numerous strains of the gastric pathogen Helicobacter pylori [11][14] . IS605 is involved in genomic rearrangements in various H. pylori isolates[35].
The H. pylori elements transpose in E. coli at detectable frequencies in a standard "mating-out" assay using a derivative of the conjugative F plasmid as a target [11][14].
The two best characterized members of this family are IS608 and the closely related ISDra2 from Deinococcus radiodurans. Both have overlapping tnpA and tnpB genes (Fig. IS200.1 ii). Like other family members, insertion is sequence-specific: IS608 inserts in a specific orientation with its left end 3’ to the tetranucleotide TTAC both in vivo and in vitro[14] while ISDra2 inserts 3’ to the pentanucleotide TTGAT[38]. Interestingly ISDra2 transposition in its highly radiation resistant Deinococcal host is strongly induced by irradiation[39] (Single strand DNA in vivo). Their detailed transposition pathway has been deciphered by a combination of in vivo studies and in vitro biochemical and structural approaches (Mechanism of IS200/IS605 single strand DNA transposition).
A more detailed and recent analysis of the distribution of 107 IS605 group elements in ISfinder is shown in Fig. IS200.4B [36]. The tree, based on TnpB sequences could be divided into 8 clusters which are overlaid onto the universal tree described by Hug et al., 2016 [37].
The IS1341 group
Elements of the third group, IS1341, are devoid of tnpA and carry only tnpB (Fig. IS200.1 v). The IS occurs in three copies in Thermophilic bacterium PS3 [8]. Multiple presumed full-length elements (including tnpA and tnpB) and closely related copies have been identified in other bacteria such as Geobacillus. On the other hand, IS891 from the cyanobacterium Anabaena is present in multiple copies on the chromosome and is thought to be mobile since a copy was observed to have inserted into a plasmid introduced in the strain[9].
Another isolated tnpB-related gene, gipA, present in the Salmonella Gifsy-1 prophage may be a virulence factor since a gipA null mutation compromised Salmonella survival in a Peyer's patch assay [40]. While no mobility function has been suggested for gipA, it is indeed bordered by structures characteristic of IS200/IS605 family ends and closely related to E. coli ISEc42.
In spite of their presence in multiple copies, it is still unclear whether IS1341 group members are autonomous IS or products of IS605 group degradation and require TnpA supplied from a related IS in the same cell for transposition.
IS decay
Circumstantial evidence based on analysis of the ISfinder database suggests that IS carrying both tnpA and tnpB genes may be unstable. Thus, although members of the IS200 group are often present in high copy number in their host genomes, intact full-length IS605 group members are invariably found in low copy number (P. Siguier, unpublished) (See also TnpB). On the other hand, various truncated IS605 group derivatives appear quite frequently (Fig. IS200.slide show 1, slide show 2 ,slide show 3, slide show 4, and slide show 5).
These forms seem to result from successive internal deletions and retain intact LE and RE copies. Sometimes, as in the case of ISSoc3 (slide show 3)., orf inactivation appears to have occurred by successive insertion/deletion of short sequences (indels) generating frameshifts and truncated proteins. For some IS (e.g. ISCco1, ISTel2, ISCysp14, ISSoc3) degradation can be precisely reconstituted and each successive step validated by the presence of several identical copies (P. Siguier, unpublished - Fig. IS200.slide show 1, slide show 2 ,slide show 3, slide show 4, and slide show 5, respectively). This suggests that the degradation process is recent and that these derivatives are likely mobilized by TnpA supplied in trans by autonomous copies in the genome.
Among the approximately 400 IS200/IS605 family entries in ISfinder (December 2023), there are more than 200 examples of IS1341-like derivatives. It was suggested that the IS1341-like derivatives might undergo transposition using a resident tnpA gene to supply a Y1 transposase in trans. There is some circumstantial evidence for transposition of IS1341-like elements. For example, IS891, present in multiple copies in the cyanobacterium Anabaena sp. strain M-131 genome [9] was observed to have inserted into a plasmid which had been introduced into the strain and more recently it has been shown experimentally that IS1341 derivatives can be mobilized by a resident tnpA gene [41] (see The IS1341 Conundrum). This can be followed from a full length IS to the formation of MITES (e.g. ISTel2; slide show 4) and MICs (e.g. ISTel3; slide show 5).
ISC: A group of Elements Related to the IS605 Group
Another group of potential IS of similar organisation, the ISC insertion sequence group, was defined by Kapitonov et al.[42] following identification of Cas9 homologues which occur outside the CRISPR structure, so called “stand-alone” homologues. While related to TnpB, they are more similar to Cas9 than to TnpB proteins. These genes were often flanked by short DNA sequences which, like LE and RE of the IS200/IS605 family, were capable of forming secondary structures. Moreover, it was reported that the ends of many ISC derivatives showed significant identity to members of the IS605 derivatives identified by these authors in the same study. (Fig. IS200.5). These structures therefore resemble the IS1341-like group.
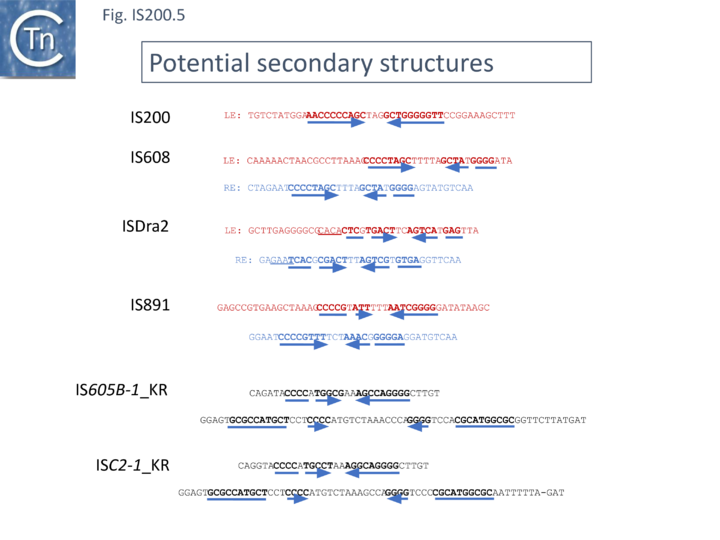
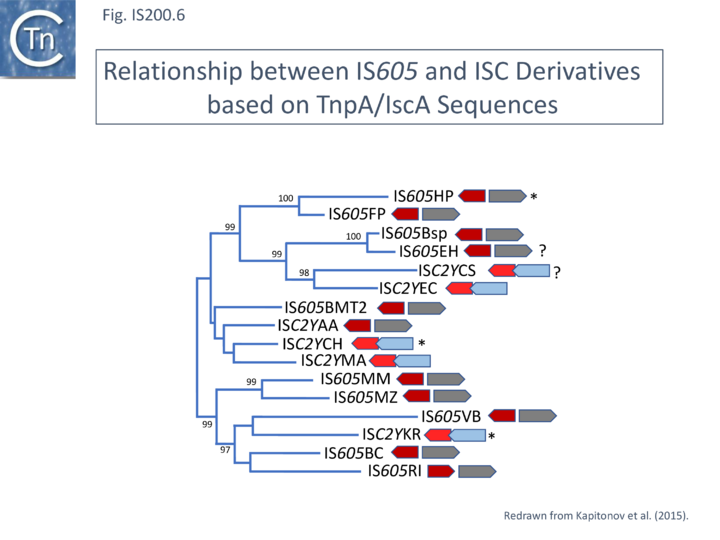
These potential transposable elements were called ISC (Insertion Sequences Encoding Cas9; not to be confused with ISCR, IS with Common Region). The name IscB was coined for the Cas9-like protein and IscA for an associated potential transposase protein which was identified in a very limited number of cases. Examples of ISC elements with both iscA and iscB genes are quite rare. Only 7 cases were identified by Kapitonov et al.,[42] (Fig. IS200.6) and only 56 of 2811 iscB examples observed in a more extensive analysis were accompanied by an iscA copy [43] . Most ISC identified were IS1341-like with only the iscB (tnpB-like) gene. These stand-alone IscB copies were identified in multiple copies in a large number of bacterial and archaeal genomes generally in low numbers (<10 copies) although some genomes contained more elevated numbers (e.g. 22 in Methanosarcina lacustris; 25 in Coleofasciculus chthonoplastes PCC 7420; 52 in Ktedonobacter racemifer)[42].
However, in contrast to the observations of Kapitonov et al.,[42] more wide-ranging studies [43] identified rare IscB proteins which were not “stand alone” but were associated with CRISPR arrays (31 examples in a sample of 2811).
A tree of “full-length” elements (Fig. IS200.6; [42])(i.e. those with both tnpA and tnpB or iscB genes) based on TnpA/IscA sequences showed that full length IS605 and ISC examples carrying both tnpA/iscA and tnpB/iscB are interleaved. IS605 is among those family members with divergent tnpA and tnpB genes (Fig. IS200.1) while other family members carry tnpA upstream of tnpB (e.g. ISDra2). However, in contrast to all IS605-like derivatives, those full length ISC elements included in this tree all have the iscA gene downstream of and slightly overlapping with iscB.
ISC have very similar transposases to those of the IS200/IS605 family and are therefore part of the same super family.
An alignment of full length TnpA from the IS200/IS605 group (Fig. 200.7; ISfinder November 2021) shows the highly conserved HuH triad, catalytic tyrosine (Y) and important glutamine (Q) residues all central to the transposition chemistry (Fig. IS200.7, Fig. IS200.11 and Fig. IS200.12) together with a number of other highly conserved amino acid positions. An alignment with the available IscA from the ISC group (Fig. 200.8 Top) shows that these also include all the highly conserved TnpA amino acid positions and are therefore very closely related to TnpA. However, the IscA and TnpA proteins appear to fall into separate clades (Fig. 200.8 bottom) with some overlap.

Since IS families are defined by their transposases rather than their accessory genes, and those of ISC and the IS200/IS605 family are so similar, it seems reasonable to include the ISC group as a subgroup of the IS200/IS605 family (or IS605 super family;[42] ). For many of the archaeal elements, there is a small, potential 40-45 amino acid, peptide located upstream of the TnpB analogue.
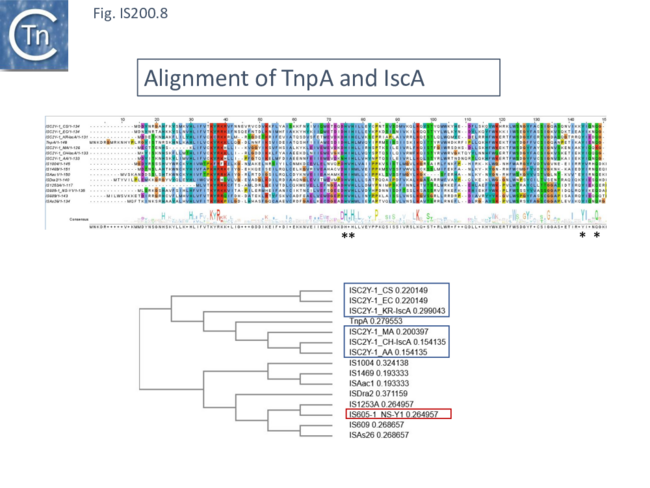
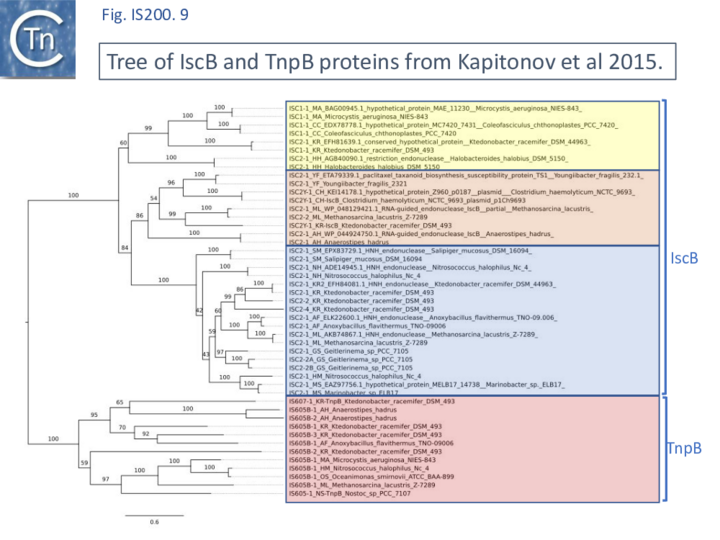
A tree based on the TnpB/IscB (Fig. IS200.9) examples presented by Kapitonov, et al.,[42] shows that the TnpB homologues form a clade separate from IscB and that the latter can be divided into two clades, IscB1 and IscB2.
These considerations therefore reinforce the idea that the IS200/IS605 family and ISC group might be considered as a superfamily which includes a number of related accessory genes (tnpB, iscB1, iscB2 etc), which carry flanking DNA sequences with secondary structure potential and in which a Y1 HuH transposase assures the chemistry of transposition. A similar conclusion was also reached by Altae-Tran et al.[43] .However, this picture is complicated by the identification of another group of transposable elements, the IS607 family in which tnpB is associated with a different type of transposase, in this case a serine site-specific recombinase (IS607 family).
Mechanism of IS200/IS605 single strand DNA transposition
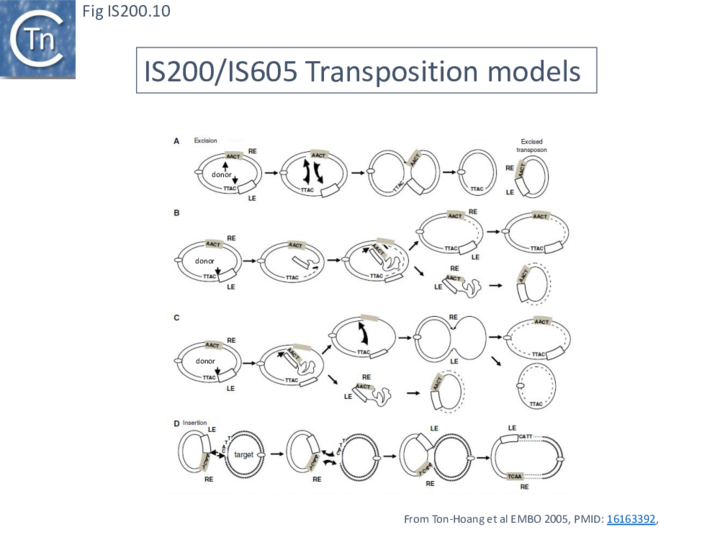
Early models
A number of alternative mechanisms were initially proposed to explain IS608 transposition [20] (Fig. IS200.10). These all included the insertion of a double-strand circular transposon copy (Fig. IS200.10 D). One model (Fig. IS200.10 A) envisaged simultaneous or consecutive cleavage at LE and RE and reciprocal strand transfer would generate a Holliday junction (HJ) which then could be resolved into double-strand circular copies of the transposon. The second (Fig. IS200.10 B) cleavage at LE and replicative strand displacement using a 3’OH of the flanking donor DNA. This could assist formation of a single strand region accessible for cleavage of RE to generate a single-strand transposon circle which could be replicated into a double-strand copy. The third (Fig. IS200.10 C) proposed cleavage at LE with displacement of the transposon strand to form a single strand loop. Subsequent in vitro and in vivo experiments (below) demonstrated that not only was IS608 capable of excision as a single-strand DNA circle but that this could be inserted into a single strand target.
General transposition pathway
The transposition pathway of IS200/IS605 family members is shown in Fig. IS200.11. Much of the biochemistry was elucidated using an IS608 cell-free in vitro system which recapitulates each step of the reaction. This requires purified TnpAIS608 protein, single strand IS608 DNA substrates and divalent metal ions such as Mg2+ or Mn2+ [20][21][22]. Similar and complementary results were also obtained with ISDra2[23][24][25]. The reactions are not only strictly dependent on single strand (ss) DNA substrates but are also strand-specific: only the “top” strand (defined as the strand carrying target sequence, TS, 5’ to the IS; Fig. IS200.11 top) is recognized and processed whereas the “bottom” strand is refractory[20] [21]. Cleavage of the top strand at the left and right cleavage sites (TS/CL and CR, note that TS is also the left cleavage site CL) (Fig. IS200.11 B) leads to excision as a circular ssDNA intermediate with abutted left and right ends (transposon joint) (Fig. IS200.11 C bottom left). This is accompanied by rejoining of the DNA originally flanking the excised strand (donor joint).
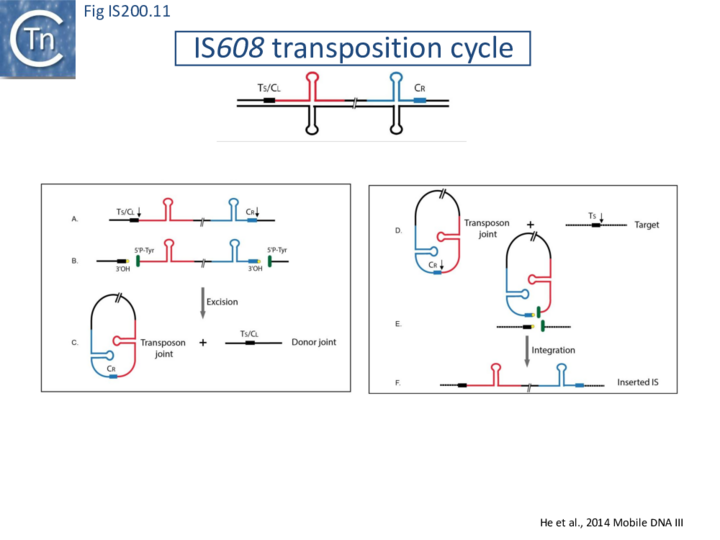
The transposon joint is then cleaved (Fig. IS200.5 E bottom right) and integrated into a single strand conserved element-specific target sequence (TS) where the left end invariably inserts 3’ to TS (Fig. IS200.5 F). This target specificity is another unusual feature of IS200/IS605 transposition. The target sequence is characteristic of the particular family member and, although it is not part of the IS, it is essential for further transposition because it is also the left end cleavage site CL of the inserted IS [20] (The Single strand Transpososome and Cleavage site recognition) and is therefore intimately involved in the transposition mechanism.
TnpA, Y1 transposases and transposition chemistry
IS200/IS605 family transposases belong to the HUH enzyme superfamily. All contain a conserved amino-acid triad composed of Histidine (H)-bulky hydrophobic residue (U)-Histidine (H)[44] providing two of three ligands required for coordination of a divalent metal ion that localizes and prepares the scissile phosphate for nucleophilic attack. HUH proteins catalyze ssDNA breakage and joining with a unique mechanism. They all catalyse DNA strand cleavage using a transitory covalent 5' phosphotyrosine enzyme-substrate intermediate and release a 3' OH group [17] (Groups with HUH Enzymes; Fig.7.5).
The HUH enzyme family also includes other transposases of the IS91/ISCR and Helitron families as well as proteins involved in DNA transactions essential for plasmid/virus rolling circle replication (Rep; not to be confused with the TnpAREP/REP system described in Domestication) and plasmid conjugation (Mob/relaxase) (Groups with HUH Enzymes; Fig.7.5).
IS200/IS605 transposases are single-domain proteins containing a single catalytic tyrosine residue, called Y1 transposase. They use the tyrosine residue (Y127 for IS608) as a nucleophile to attack the phosphodiester link at the cleavage sites (vertical arrows in Fig. IS200.11 A and D). Since cleavages at both IS ends occur on the same strand, the polarity of the reaction implies that the enzyme forms a covalent 5’-phosphotyrosine bond with the IS at LE producing a 3’-OH on the DNA flank and a 5’-phosphotyrosine bond at the RE flank producing a 3’-OH on RE itself (Fig. IS200.11 B). The released 3′-OH groups then act as nucleophiles to attack the appropriate phospho-tyrosine bond resealing the DNA backbone in one case and generating a single-strand DNA transposon circle in the other (Fig. IS200.11 C). The same polarity is applied to the integration step (Fig. IS200.11 D, E and F). As an important mechanistic consequence of this chemistry, IS200/IS605 transposition occurs without loss or gain of nucleotides. In vitro, the reaction requires only TnpA and does not require host cell factors.
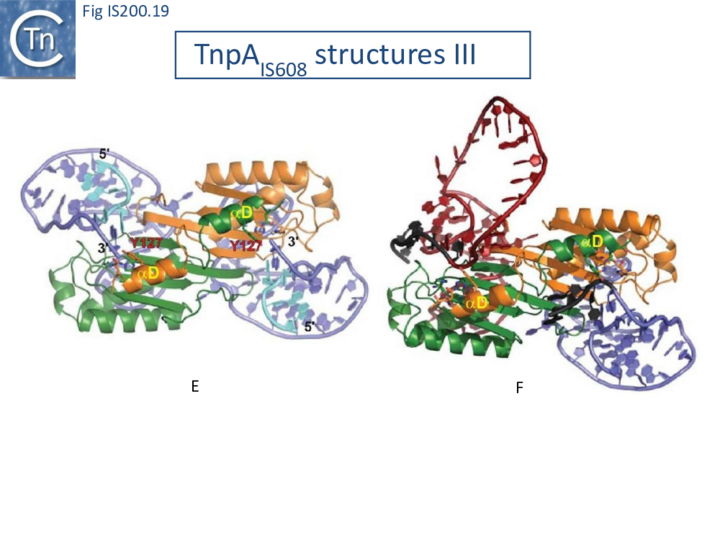
TnpA overall structure
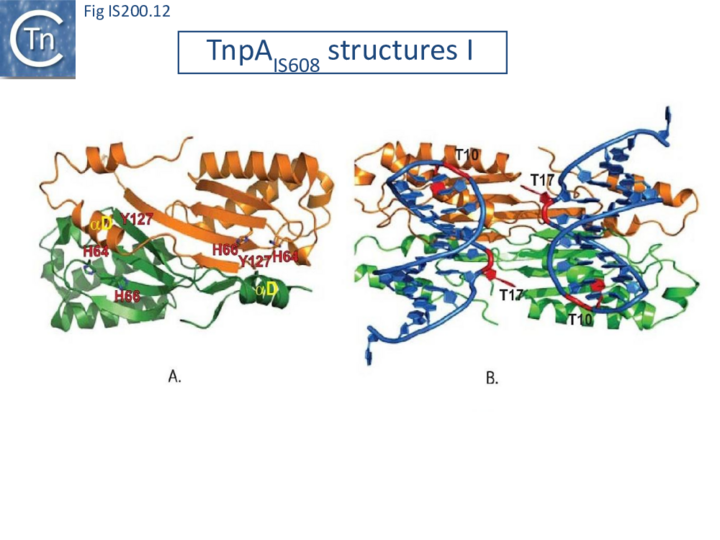
Crystal structures of Y1 transposases have been determined for three family members: IS608 (TnpAIS608) from Helicobacter pylori [19][22] ISDra2 (TnpAISDra2) from Deinococcus radiodurans [25] and ISC1474 from Sulfolobus solfataricus[45]. In contrast to most characterised HUH enzymes, which are usually monomeric and have two catalytic tyrosines, Y1 transposases form obligatory dimers with two active sites (Fig. IS200.12 A). The two monomers dimerize by merging their β-sheets into one large central β-sheet sandwiched between α-helices. Each catalytic site is constituted by the HUH motif from one TnpA monomer (H64 and H66 in the case of TnpAIS608) and a catalytic tyrosine residue (Y127) located in the C-terminal αD helix tail of the other monomer (Fig. IS200.12 A). This is joined to the body of the protein by a flexible loop (trans configuration, Active site assembly and Catalytic activation and Transposition cycle: the trans/cis rotational model).
The TnpA enzyme active sites are believed to adopt two functionally important conformations: the trans configuration described above (Fig. IS200.12 A), in which each active site is composed of the HUH motif supplied by one monomer with the tyrosine residue supplied by the other, and the cis configuration, in which both motifs are contributed by the same monomer (IS200/IS605 video 1 below; kindly supplied by O. Barabas and Fred Dyda).
The trans conformation is active during cleavage where Tyrosine acts as nucleophile whereas the cis conformation is thought to function during strand transfer where the 3’OH is the attacking nucleophile (Transposition cycle: the trans/cis rotational model). Only the trans configuration of TnpAIS608 and TnpAISDra2 has yet been observed crystallographically [19][25] but the existence of the cis configuration is supported by biochemical data [46].
IS200/IS605 video 1
|
|---|
The Single strand Transpososome
The key machinery for transposition is the higher-order protein-DNA complex, the transpososome (or synaptic complex) which contains both transposase and two IS DNA ends with or without target DNA. Transpososome formation, stability, and the temporal changes in a configuration which occur during the transposition cycle have been characterized for TnpAIS608 by crystallographic and biochemical approaches.
Although for technical reasons it was not possible to obtain structures with both LE and RE hairpins together, co-crystal structures with either LE or RE showed that a TnpA dimer binds two subterminal DNA hairpins suggesting that it could bind both LE and RE ends simultaneously. Binding sites for the hairpins are located on the same face of the TnpA dimer while the two catalytic sites are formed on the opposite surface (Fig. IS200.6 A and B) (IS200/IS605 video 2 below; kindly supplied by O. Barabas and Fred Dyda). The hairpin forms a distorted helix anchored by base interactions at the foot (IS200/IS605 video 2 below; kindly supplied by O. Barabas and Fred Dyda).
IS200/IS605 video 2
|
|---|
Substrate recognition
A key feature of TnpA is that it is only active on one strand, the “top” strand. The IS608 and ISDra2 ends carry subterminal imperfect hairpins. In addition to specific sequences on the loops, the irregularities on the hairpins help the enzyme to distinguish between “top” and “bottom” strands [19][25]. The initial co-crystal structure was obtained with TnpAIS608 and a 22nt imperfect RE hairpin (HP22) including its characteristic extrahelical T17 located mid-way along the DNA stem (Fig. IS200.12 and Fig. IS200.13). In addition to a number of backbone contacts with HP22, TnpAIS608 also shows several base-specific contacts, in particular with T10 in the loop and the extrahelical T17[19] (Fig. IS200.12 B).
Exchange of T10 and neighboring T nucleotides in the loop abolished binding whereas the exchange of T17 for an A significantly reduced but did not eliminate binding [47]. Similar studies with TnpAISDra2 showed that it also recognises a similarly located T in the hairpin loop of ISDra2 and that this is essential for binding [25] . Instead of an extrahelical T, ISDra2 LE and RE include a bulge caused by two mismatched nucleotides (G and T) in the hairpin stem. These unpaired nucleotides are specifically recognized and stabilized by the protein. Again, mutation of the T (to C which, in this case, eliminates the bulge to generate a GC base pair in the stem) greatly reduces binding (IS200/IS605 video 3A below; kindly supplied by O.Barabas and Fred Dyda).
Although most members of the IS605 group, which includes IS608 and ISDra2, have imperfect palindromes with extrahelical bases or bulges, some members of the IS200 group (e.g IS200, IS1541) include perfect hairpins. Whether base-specific interactions with the loop sequence is exclusively responsible for strand-specific activity of the corresponding transposase remains to be clarified.
IS200/IS605 video 3A
|
|---|
Cleavage site recognition
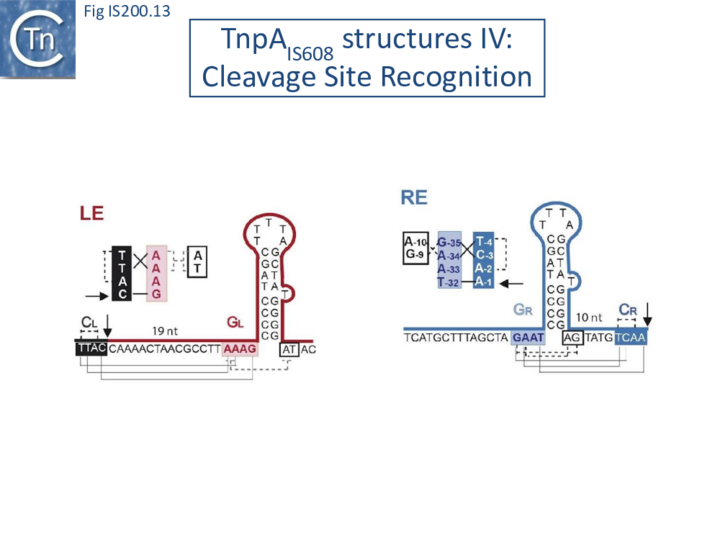
The left (CL/TS) and right (CR) IS608 cleavage sites (TTACl and TCAAl respectively, where l represents the point of cleavage) are located some distance from the subterminal recognition hairpins (19 nt at LE and 10 nt at RE) (Fig. IS200.13). The system is asymmetric because the two distinct cleavage sites are separated from the hairpins by linkers of different lengths and the CL/TS sequence does not form part of IS while CR does.
Structural studies revealed that the cleavage sites are recognized in a unique way that does not involve direct sequence recognition by TnpA. Instead, an internal part of the IS sequence is co-opted to recognize different cleavage sites allowing TnpA to catalyze both excision and integration of the element with a single DNA binding domain.
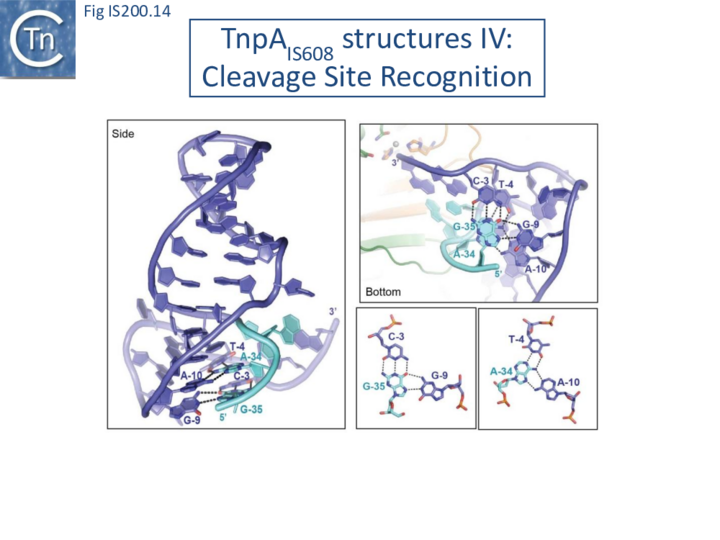
Internal transposon sequences, the left (GL) and right (GR) tetranucleotide guide sequences, AAAG and GAAT, located 5’ to the foot of the hairpins (Fig. IS200.7), recognize their respective cleavage sites by direct base interactions. These GL/CL and GR/CR interactions involve 3 of the 4 nt of GL and GR. They include both canonical Watson-Crick interactions and in the case of RE, non-canonical interactions resulting in base triplets (Fig. IS200.13 and Fig. IS200.14, bases joined by both regular and dotted lines respectively). In the case of LE and the transposon joint, base triples (dotted lines) are suggested from biochemical data [47] (IS200/IS605 video 3B below; kindly supplied by O. Barabas and Fred Dyda).
IS200/IS605 video 3B
|
|---|
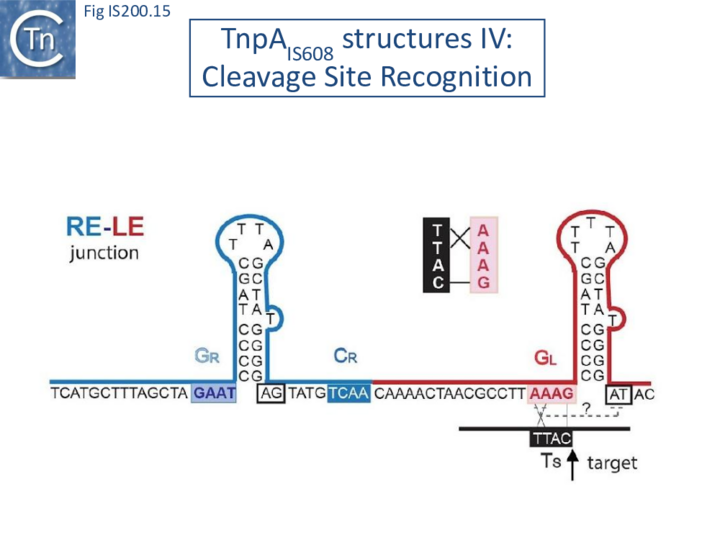
These interactions place the scissile phosphate precisely into the two active sites of TnpAIS608 for nucleophilic attack by the catalytic Y127. Interestingly, the base-pairing patterns responsible for cleavage site recognition are similar at LE, RE and the target site in spite of sequence differences (Fig. IS200.13, Fig. IS200.14, Fig. IS200.15). Since TS is also CL, this type of recognition not only explains the requirement for the TS located at the left end of the inserted IS (Fig. IS200.11, Fig. IS200.15) for further transposition, but also the target specificity. Upon integration, TS is presumably recognized by the GL present on the excised transposon joint. Note that the transposon joint contains only the LE guide sequence GL but not the LE cleavage site CL (Fig. IS200.11, Fig. IS200.15).
Similar crystal structures were obtained with TnpAISDra2 (see also Single strand DNA in vivo) with a similar interaction network between the guide sequences and cleavage sites.
The ISDra2 transpososome is structurally very similar to those of IS608 despite only 34% sequence identity of the transposases. It is important to note that the target sequence in ISDra2 is a pentanucleotide instead of a tetranucleotide as in IS608. The fifth nucleotide in the ISDra2 sequence is however not involved in DNA-DNA interactions but in DNA-protein interaction[25].
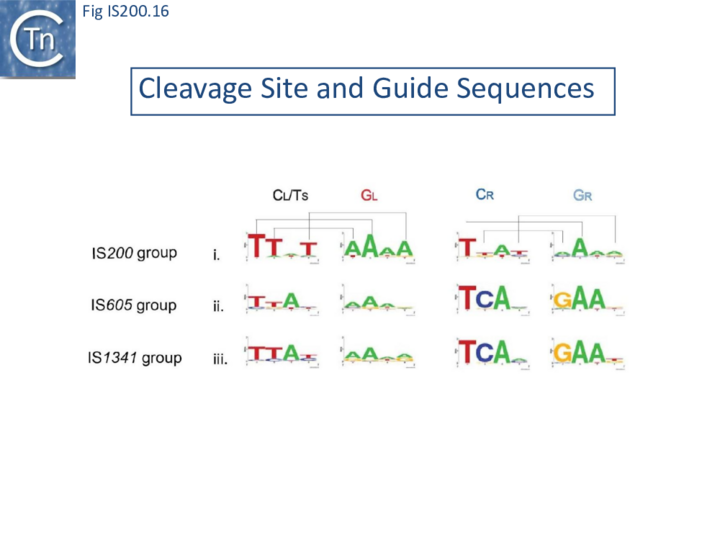
The potential cleavage site recognition mode (i.e. the canonical interaction network between CL,R and GL,R) is indeed well conserved throughout the family (Fig. IS200.16).
This model has been validated in vitro and in vivo by showing that it is possible to modify cleavage sites by changing corresponding guide sequences. Moreover, in the case of IS608, modifications of GL in the transposon joint generate predictable changes in insertion site-specificity of the element [48]. The IS608 recognition system has also been modified to include additional sequences which assist more specific targeting of insertions[49].
Active site assembly and Catalytic activation
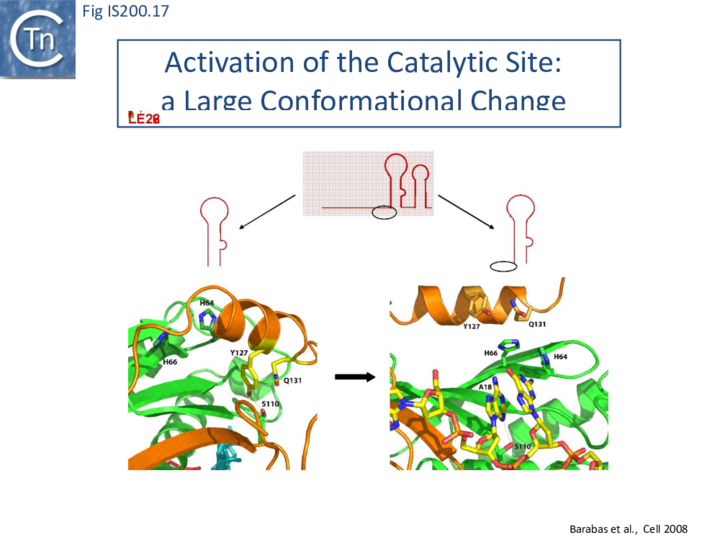
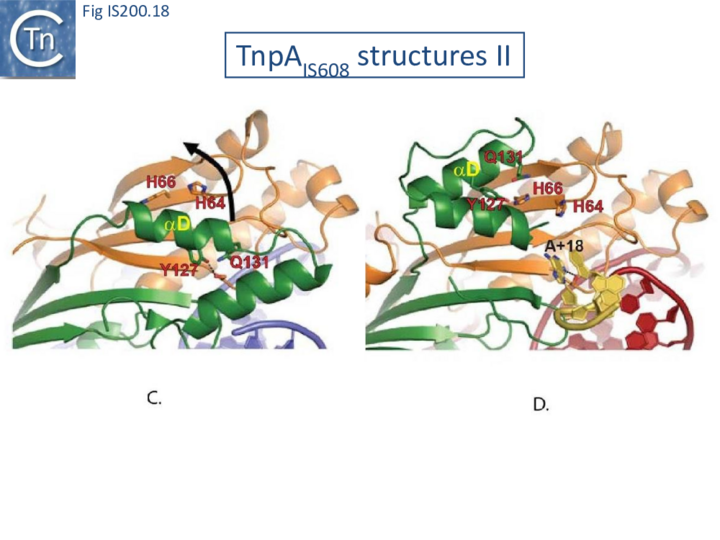
Comparison of crystal structures of different TnpA protein-DNA complexes [19][22] [45] revealed TnpA in both active and inactive configurations. In both the free TnpAIS608 dimer and TnpAIS608-DNA complexes bound to a “minimal” HP22 hairpin (which does not include the guide sequence), the catalytic tyrosine residue (Y127) points away from the HUH motif (H64 and H66) and therefore cannot act as a nucleophile [19] (Fig. IS200.11).
The enzyme is therefore in an inactive conformation. Binding to the appropriate substrate containing the 4 nucleotide guide sequence 5’ to the hairpin foot (compare Fig. IS200.17 left and right) triggers a change in TnpA configuration that permits assembly of functional active sites. A single A (A+18, Fig. IS200.13 and Fig. IS200.13) in the guide sequence present in both GL and GR does not participate in base interactions with the cleavage site. On formation of the CL(R)/GL(R) base interaction network, this single base penetrates the structure and forces the C-terminal αD helix carrying Y127 closer to the HuH motif placing it in the correct position poised for catalysis [22] (compare Fig. IS200.17 left and right; Fig. IS200.18)(IS200/IS605 video 4 below; kindly supplied by O. Barabas and Fred Dyda).
This movement also places a third amino acid (Q131 located at the C-terminal end of helix αD on the same face as Y127) in a position enabling it to function in conjunction with both H residues to complete the metal ion binding pocket. This movement is made possible by the fact that the αD helix is attached to the protein body by a flexible loop. This conformational change involving αD helix movement will be discussed below (Transposition cycle: the trans/cis rotational model).
IS200/IS605 video 4
|
|---|
Transpososome assembly and stability
Excision requires the assembly of a transpososome containing both LE and RE. However, it is technically difficult to generate crystallographically pure complexes of this type. Only crystal structures containing two LE or two RE were obtained. The excision transpososome was initially modelled using information obtained from the IS608LE-TnpA and RE-TnpA structures [22] (Fig. IS200.12 B; Fig. IS200.19). However, complexes containing both LE and RE have now been identified using a band shift assay and characterized biochemically [47].
A TnpA co-complex with either LE or RE can be titrated by the addition of increasing quantities of the other end (RE or LE) to obtain a transpososome containing both LE and RE. This can be easily detected in a gel shift assay. Such species proved to be catalytically active since they could be removed from the gel and, when incubated with the essential divalent metal ion, robust reaction products could be detected in a denaturing ge [47].
This approach was used to monitor both transpososome formation and stability using oligonucleotides carrying point mutations in GL,R and CL,R. Robust transpososome formation and cleavage activity requires much of the network of GL,R and CL,R interactions observed in the crystal structures [47] (schematised in Fig. IS200.13). Although base triplets in the original LE co-crystal structure were not detected since the LE substrate was too short [22], the biochemical data suggested that such interactions probably exist (grey dotted lines in Fig. IS200.13).
For example, the two nucleotides 3’ to the foot of the LE hairpin (at equivalent positions to triplet forming bases in RE, Fig. IS200.13 are required for robust synaptic complex formation and cleavage [47]. This further implies that these base triplets might also be involved in target DNA capture (grey dotted lines in Fig. IS200.15).
Base changes in GL resulted in a predictable choice of target sequence [48]. However, large differences in insertion frequencies were observed. The influence of the presumed non-canonical interactions in LE would provide an explanation for this variability since these were not taken into account in the choice of LE guide sequence.
In both IS608 and ISDra2, the extra-helical bases in the hairpin stem and nucleotides in the loop are also important for transpososome formation even in a context which includes both GL,R and CL,R[25][47].
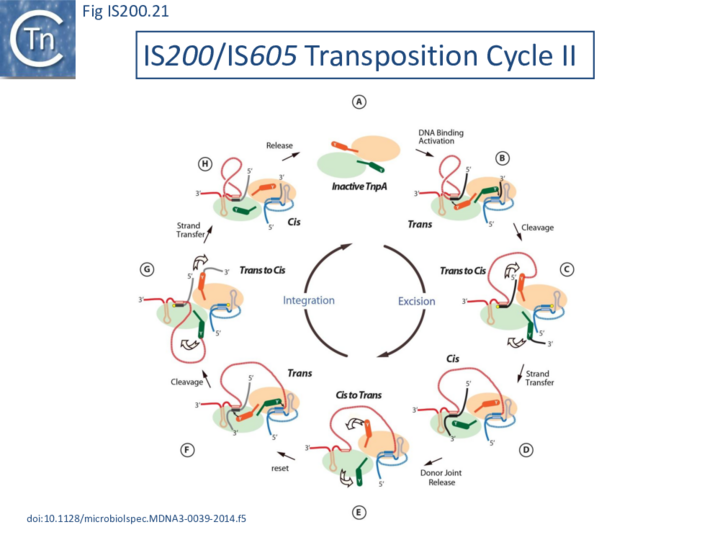
Transposition cycle: the trans/cis rotational model
Transpososome assembly is followed by two critical chemical steps: cleavage and strand transfer. These are thought to be accomplished by a series of large changes in transpososome configuration. A detailed model has been proposed for the dynamics of the IS608 transpososome during the transposition reactions[22] (Fig. IS200.19; IS200/IS605 video 1). As described in TnpA overall structure (above), TnpAIS608 could in principle assume two configurations: trans and cis. Switching between these two states would involve rotation of the two unconstrained flexible arms which join the αD helix to the protein body.
The current model for IS608 and ISDra2 transposition proposes that the strand transfer step involves rotation of these arms from the trans to the cis configuration: cleavage occurs while the enzyme is in the trans configuration. A trans to cis conformational change then occurs allowing strand transfer. The ground state of the IS608 and ISDra2 transpososomes obtained from crystallography is the trans configuration. LE and RE binding and cleavage occur with the enzyme in its trans configuration (Fig. IS200.19; IS200/IS605 video 1).
This results in the formation of the 5’ phosphotyrosine bond with LE liberating a 3’-OH on the flanking DNA and the 5’phosphotyrosine bond with the RE DNA flank liberating a 3’-OH on the RE transposon end. Rotation of the two arms would displace LE towards the sequestered 3’-OH of RE and the RE flank towards the 3’-OH of the LE flank (Fig. IS200.19; IS200/IS605 video 1) and position them so that both 3’-OH can attack the appropriate phosphodiester bond. This model is supported by several lines of indirect evidence from studies of IS608.
An initial piece of evidence concerns the length differences in the LE and RE “linker” (the distance between the hairpin foot and the cleavage site): this is only 10 nt for RE but 19 nt for LE (Fig. IS200.15). The rotation model suggests that the longer LE linker may be required to provide sufficient length to rotate the 5’ LE phospho-tyrosine bond to position it closes the immobile RE 3’-OH (Fig. IS200.19; IS200/IS605 video 1). This would imply that LE linker length is critical for strand transfer. Indeed, sequential reduction in the length of the LE linker has a large effect on transposition frequency and excision in vivo. In vitro, it also had a somewhat larger effect on strand transfer than on cleavage [47], supporting the idea that the linker is important for mechanical movement.
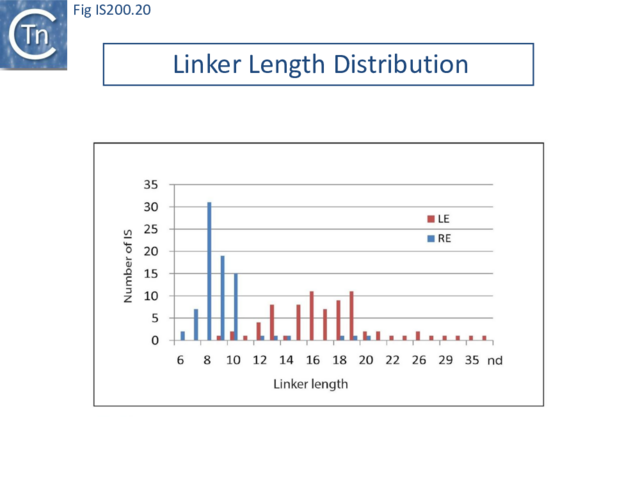
However, transpososome formation and stability was also observed to be affected with the shortest linkers. This presumably reflects steric barriers to GL(R)/CL(R) interaction and supports the notion that these interactions are important in transpososome assembly. A survey of over 100 different IS from all three groups (35 from the IS200 group; 47 from IS605 and 24 from IS1341) in the public databases has shown that the asymmetry of the IS608 ends is conserved across the entire family: the left linker is always longer than the right (15-16 nt versus 8 nt) [46] (Fig. IS200.20).
The second piece of evidence comes from the behaviour of TnpAIS608 heterodimers carrying point mutations in the HuH or catalytic Y. These were expressed and assembled in vivo and purified based on two different C-terminal affinity tags (one for each monomer). This permitted heterodimers to be distinguished form homodimers. A heterodimer with a combination of mutations that enforce a trans-active TnpA site (in which the wildtype HuH motif and Y127 belong to different TnpA monomers) is proficient for cleavage but not for rejoining. In contrast, a heterodimer with cis-active TnpA site (in which the wildtype HuH motif and Y127 belong to the same TnpA monomer) is proficient for rejoining but inactive in cleavage [46].
This implies that all chemical reactions involved in cleavage occur in the trans site while the chemical reactions for strand transfer occur in the cis site. This strongly supports the rotational model.
A third piece of evidence comes from studies of the flexible arm that joins helix αD to the body of the protein and which is proposed to play a pivotal role in the rotation. This flexibility may be facilitated by two glycine residues (G117 and G118). Mutation of these two residues did not affect strand cleavage but led to inhibition of strand transfer suggesting that the two residues are required for achieving a cis configuration. The importance of these G residues is reflected in their conservation throughout the family [46].
Thus, while the cis configuration has not been observed crystallographically for these elements, its existence is strongly suggested by experimental data, supporting the trans/cis rotational model (Fig. IS200.21).
Regulation of single strand transposition
Single strand DNA in vivo
The obligatory single-stranded nature of IS200/IS605 transposition in vitro suggests that it is limited in vivo by the availability of its ssDNA substrates inside the cells and processes that produce ssDNA may stimulate transposition. We describe below a link between the transposition of these elements and the replication fork. Moreover, in the case of ISDra2, single strand DNA produced during re-assembly of the D. radiodurans genome following irradiation results in stimulation of transposition[23][50]. Transcription or other processes leading to horizontal gene transfer such as transformation, conjugative transfer, or transduction with single strand phages might also favor their mobility.
Replication fork
The replication fork modulates the transposition of many transposable elements (Tn7, IS903, IS10, IS50, Tn4430, P element[51][52][53][54][55][56]. For IS200/IS605 family members, the replication fork, in particular the lagging strand template, is an important source of ss DNA substrates for both excision and integration. Transposition can be considered to follow a “Peel and Paste ” mechanism (Fig. IS200.22) where the IS excises or is “peeled” off as a single strand circle from the lagging strand template of the donor molecule and then integrates or is “pasted” in a ss target at the replication fork.

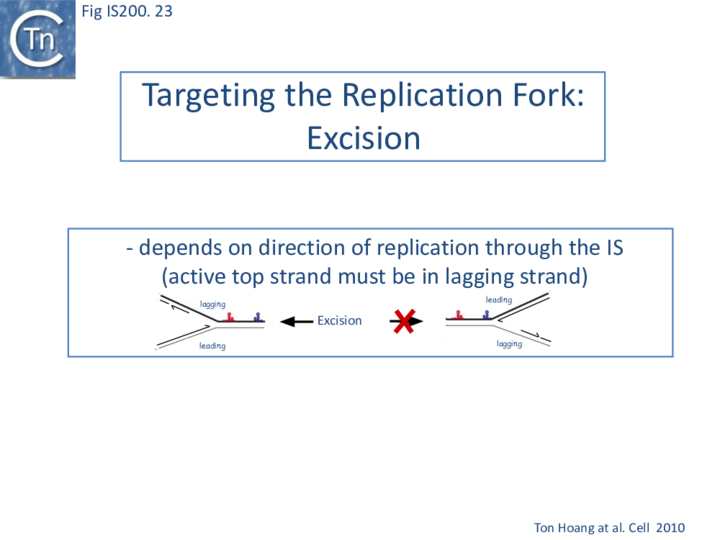
Excision: Excision of IS608 is sensitive to the direction of replication across the element: it is more frequent when the active strand (top strand) is on the lagging strand (discontinuous) template (Fig. IS200.22 top; Fig. IS200.23) but difficult to detect when it is on the leading (continuous) strand [24]. Moreover, excision in vitro requires that both ends are in single strand form at the same time[20].
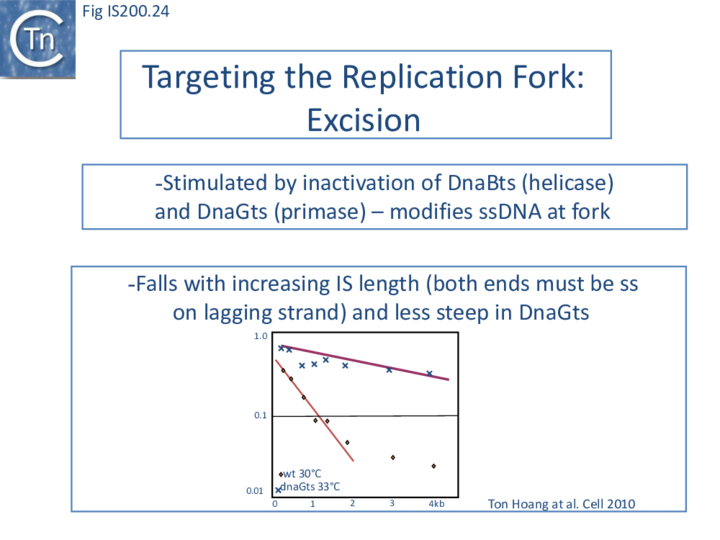
The length of ssDNA on the lagging-strand template depends on the initiation frequency of Okazaki fragment synthesis by the DnaG primase[57][58]. Transient inactivation of DnaG activity reduces this frequency and therefore increases the average length of ssDNA between Okazaki fragments; the IS608 excision frequency increased. Under permissive conditions for E. coli carrying a dnaGts mutation, using a plasmid-based assay with IS608 derivatives of different lengths, the excision frequency decreased strongly as IS length increased. In contrast, when DnaGts activity was reduced by growth under sub-lethal conditions, excision showed a much less pronounced length-dependence (Fig. IS200.24). This length-dependence might also contribute to the difference in copy numbers observed in the IS200 and IS605 groups (see "Distribution and Organization").
Integration: IS608 integration is oriented (with its left end 3’ to a TTAC target site) and it requires an ssDNA target in vitro [14][21]. The close link between transposition and the replication fork is also illustrated by the integration bias, consistent with a preference for an ssDNA target on the lagging strand template (Fig. IS200.22 bottom). This was indeed found to be the case in E. coli for both plasmid and chromosome targets [24]. As expected, the orientation of insertions into the E. coli chromosome was correlated with the direction of replication of each replicore and was consistent with integration into the lagging strand template.
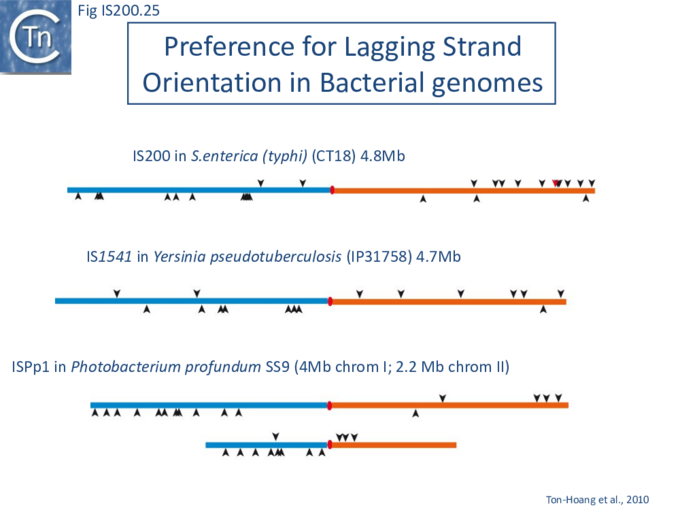
The orientation bias is not restricted to [3]IS608 and ISDra2. An in silico analysis of a large number of bacterial genomes carrying copies of various family members revealed that most had a strong insertional bias consistent with the direction of replication[24] (Fig. IS200.25). Moreover, in certain cases, elements which did not follow the orientation pattern could be correlated to the genomic region that had undergone inversion or displacement (Fig. IS200.26; Fig. IS200.27) suggesting that, once they occur, insertions are quite stable. It seems possible that this type of genomic archaeology based on orientation patterns could be used to complement the study of bacterial genome evolution.
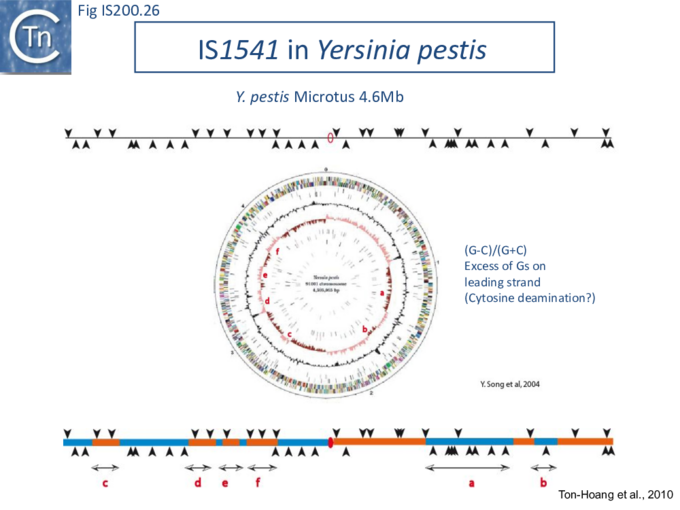
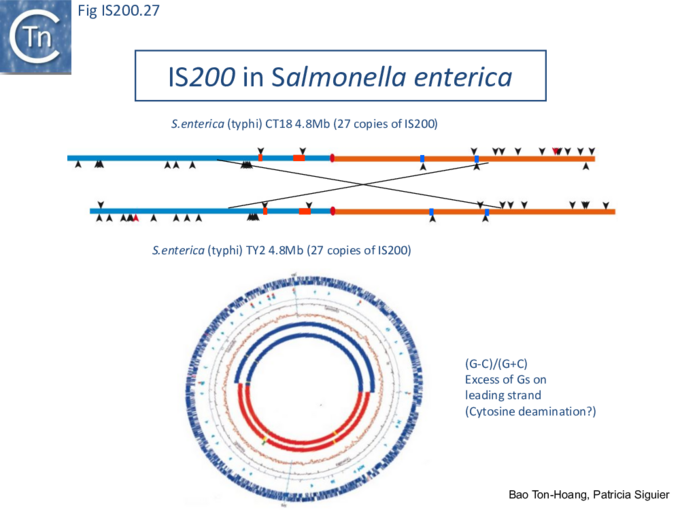
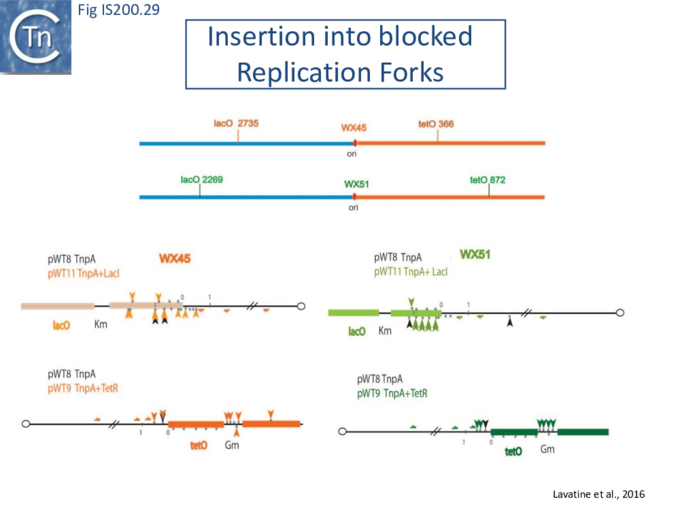
Stalled replication forks: Stalled replication forks appeared preferential targets for IS608 insertion. In the experiments using the Tus/ter replication termination or operator/repressor system, replication fork arrest attracts IS608 insertion [24]. Transient blockade of the unidirectional replication fork by the Tus protein at the ter site resulted in preferential IS608 insertion into the array of target sequences behind the stalled forks on the lagging strand but not on the leading strand (Fig. IS200.28). A similar result was obtained in the E. coli chromosome using the lacI/lacO and tetR/tetO repressor/operator roadblock systems[59][60] (Fig. IS200.29). Moreover, a significant number of IS608 insertions into the E. coli chromosome were localized in the highly transcribed rrn operons. This suggests that high transcription levels might affect replication fork progression (fork arrest by collision with RNA polymerase, R-loop formation, etc.) and could account for targeting the rrn operons. Thus, IS608 insertions can be targeted to the stalled forks and this may well represent a major pathway for targeting transposition.

Genome re-assembly after irradiation in Deinococcus radiodurans
Deinococcus radiodurans, arguably the most radiation-resistant organism known, has a remarkable capacity to survive the lethal effects of DNA-damaging agents, such as ionizing radiation, UV light and desiccation. After exposure to high irradiation doses, the D. radiodurans chromosome which is present in multiple copies per cell[61][62] is shattered and degraded, but can be very rapidly reassembled in a process called ESDSA (Extended Synthesis Dependent Strand Annealing). This involves resection of the multiple dsDNA fragments to generate extensive ssDNA segments, reannealing of complementary DNA and reconstitution of the intact chromosome [39].
Mennecier et al.[50] analyzed the mutational profile in the thyA gene following irradiation. The majority of mutants were due to the insertion of a single IS, ISDra2 which is present in a single copy in the genome of the laboratory D. radiodurans strain. Furthermore, using a tailored genetic system, both ISDra2 excision and insertion efficiency was found to increase significantly following host cell irradiation[23]. A PCR-based approach was used to follow irradiation-induced excision of the single genomic ISDra2 copy and re-closure of flanking sequences. Remarkably, these events are temporally closely correlated with the start of the ESDSA. The signal that triggers ISDra2 transposition is likely the production of ssDNA intermediates generated during genome reassembly. Consistent with this, the requirement of ssDNA substrates for ISDra2, as for IS608, was confirmed by in vitro studies of TnpAISDra2-catalysed cleavage and strand transfer[23].
ISDra2 excision also depends on the direction of replication and is consistent with a requirement for the active strand to be located on the lagging strand template in normally growing cells. However, this bias disappeared in irradiated D. radiodurans [24]. Since no apparent strand bias was observed in generating ssDNA during ESDSA, the lack of orientation bias in irradiated D. radiodurans suggests that ssDNA substrates are no longer limited to those rendered accessible during replication. This indicates that ssDNA sources are different in the contexts of vegetative replication and in genome reassembly.
Real-time transposition (excision) activity
The dynamics of IS608 excision from a donor site has been examined at the colony and single-cell level in real-time using an artificial IS608 derivative inserted between the -35 and -10 elements of a PlacIQ1 promoter[63] driving expression of the blue fluorescent protein mCerulean[64]. TnpAIS608, N-terminally tagged with the bright yellow reporter Venus[65] was supplied in trans driven by PLTetO1 and controllable over a 100x range. Excision rates were proportional to the transposase levels and, as expected, excision depended on the orientation of the IS derivative with respect to the direction of replication in the donor plasmid: IS in an orientation with the active IS strand in the lagging strand template excised more frequently and at lower (10x) TnpA levels than when inserted into the leading strand, demonstrating the validity of the experimental system. In this system, individual excision events as bright flashes of blue fluorescence. Following an initial activity in the part of the population when cells are applied to a solid medium, activity decreases or ceases during “exponential” growth but increases again at a constant rate (in a sub-population) upon growth arrest in a random (Poisson distributed) way. Moreover, the events do not occur randomly in the growing colonies and tend to be excluded from the colony edges. The study underlines the heterogeneity of TE activity rates in both space and time possibly resulting from heterogenous TnpA levels at the individual cell level in the population. These studies are reminiscent of the early studies of Jim Shapiro on phage Mu-mediated rearrangements in growing bacterial colonies[66][67].
TnpB and its Relatives: Guide RNA Endonucleases
TnpA alone can carry out both the cleavage and joining steps in vitro. TnpB is encoded only by the IS1341 and IS605 groups and is not required for transposition of either IS608 or ISDra2 in Escherichia coli and Deinococcus radiodurans respectively [14][20]. The full length TnpB is approximately 400 amino acids long.
IS200/IS605 and the ISC group
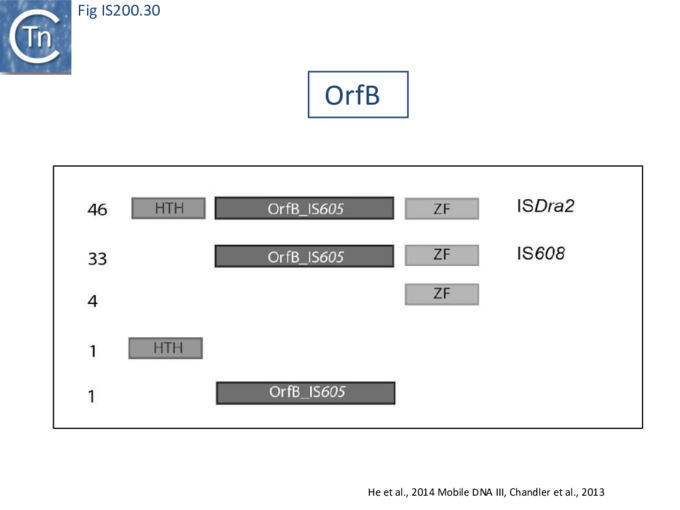
An overview of TnpB organization was originally obtained by comparing the entire ISfinder collection of 85 tnpB copies with the Pfam domain database (Fig. IS200.30). This revealed three major domains: an N-terminal putative helix-turn-helix, a longer and more variable central domain, OrfB_IS605, with a putative DDE motif and a C-terminal zinc finger (ZF) domain of the CPXCG type. Half of the analyzed TnpB copies including TnpBISDra2 but not TnpBIS608 contained all three domains, while only two did not include a zinc finger.
TnpBIS608 was missing the N-terminal HTH domain which would provide an explanation for its lack of activity in certain assays [41].
Pasternak et al.[68] observed that TnpBISDra2 appears to have an inhibitory effect on ISDra2 excision and insertion in its host, D. radiodurans, and on excision in E. coli, and that the integrity of its putative zinc finger motif is required for this effect.
Relatives of TnpB has been identified in both prokaryotes and eukaryotes. It is carried by members of the IS607 family found both in prokaryotes and in eukaryotes and their viruses but is dispensable for IS607 transposition in E. coli . As it is for IS200/IS605 transposition. TnpB analogues, known as Fanzor1 and Fanzor2 (see: Fanzor section below), have also been identified in diverse eukaryotic transposable elements.
TnpB and IscB are Related to the RNA-guided nucleases Cas12 and Cas9.
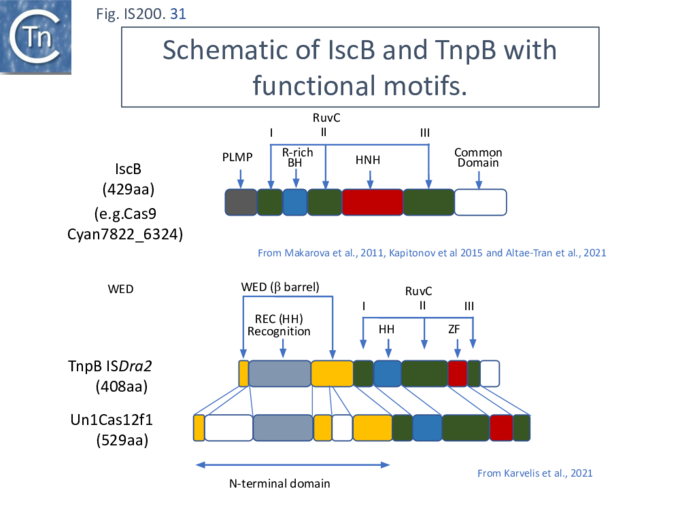
More extensive analysis showed that TnpB shares some similarity with the RNA-guided nuclease Cas12 while IscB showed greater similarity to Cas9. Both, like Cas9 and Cas12, themselves exhibit split RuvC endonuclease domains [42][69] [70][71][72] (Fig. IS200.31). While Cas9 and Cas12 carry related functional domains, their architectures are somewhat different and the configuration of their guide RNAs also differ.
IscB and Cas9
Cas9 (also called Cas5, Csn1, or Csx12) is an RNA-guided dual nuclease generally associated with CRISPR systems in bacteria and widely used in genome engineering. The RuvC DED catalytic triad is split into three sections (I, II and III) in which I and II are interrupted by the R-rich region and II and III by an HNH nuclease domain (Fig. IS200.31). A region common to all Cas9 derivatives is located at the C-terminal end.
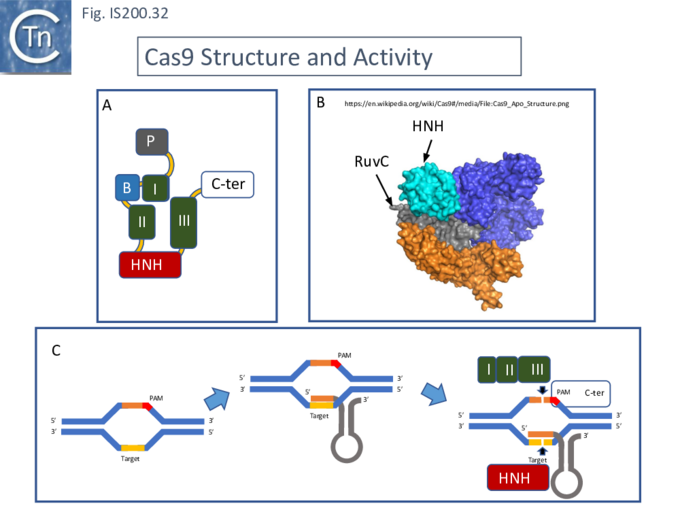
The Cas9 structure has been determined (Fig. IS200.32. B [73]). The protein is a monomer in which the three RuvC segments I, II and II carrying the D, E and D catalytic residues respectively, are assembled into the correct three-dimensional configuration to generate a RuvC-like catalytic pocket with the HNH nuclease domain extruded (Fig. IS200.32. A). The Cas9 guide RNA (crRNA) is composed of a region containing secondary structure potential and a 5’ extension (spacer) of about 20 nts, complementary to the target sequence and which forms an RNA/DNA heteroduplex (Fig. IS200.32. C). Activated Cas9 recognises a specific sequence, PAM (Protospacer Adjacent Motif), located next to the target sequence on the complementary strand downstream of the target sequence. This is necessary for binding of the Cas9-crRNA complex and subsequent cleavage [74]. Cleavage is catalysed by both the HNH nuclease (target strand) and the reconstituted RuvC nuclease (complementary strand). Cleavage is often “blunt” (i.e. occurs at the same position on both strands) and PAM proximal [74].
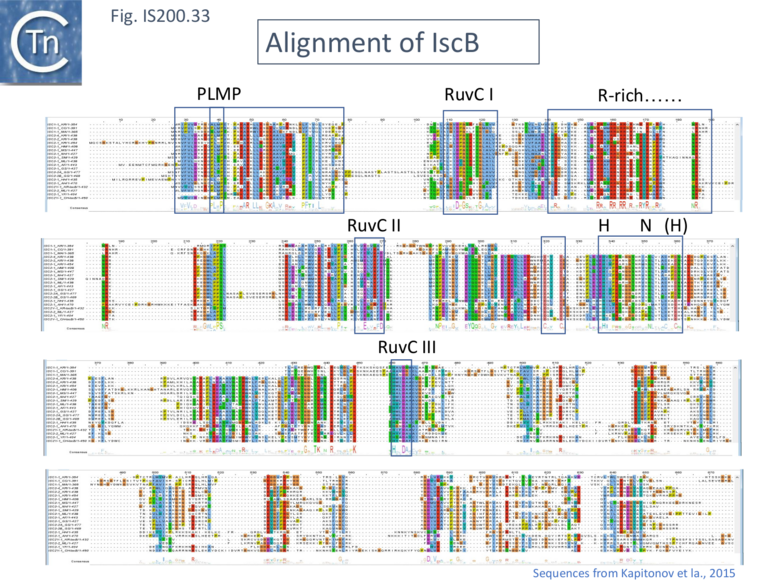
IscB shares Cas9 sequence features such as the split RuvC and HNH nuclease domains and an arginine-rich (R-rich also known as a bridge helix) domain (Fig. IS200.31 Top) with a group of Cas9 derivatives, Cyan7822_6324, in particular [75]. In addition, a more detailed investigation [43] led to identification of an additional IscB N-terminal domain (called PLMP after its conserved amino acid residues) not present in Cas9 (Fig. IS200.30. Top). These features appear in alignments of IscB sequences [42] ; Fig. IS200.33.
TnpB and Cas12
Cas12 is also an RNA-guided nuclease. A number of subtypes have been described [76] and the structures of several of these have been solved. They have similar C-terminal ends but carry (related) N-terminal ends of various lengths (see Karvelis, et al.[72]). One of the shorter derivatives Cas12F (AKA Cas14) [77] acts as a dimer. Like Cas9, the common C-terminal end is composed of a split RuvC (I, II and III) in which I and II are interrupted by the R/K-rich region. In this case, however, instead of the HNH domain, RuvC segments II and III are separated by a zinc finger of the CPXCG typeI (Fig. IS200.31 bottom).
For Cas12, the guide RNA is composed of a region containing secondary structure potential and a 3’ extension (spacer) of about 20 nts, complementary to the target sequence (Fig. IS200.34). The PAM sequence is located upstream of the target sequence. Cleavage is PAM distal and staggered.
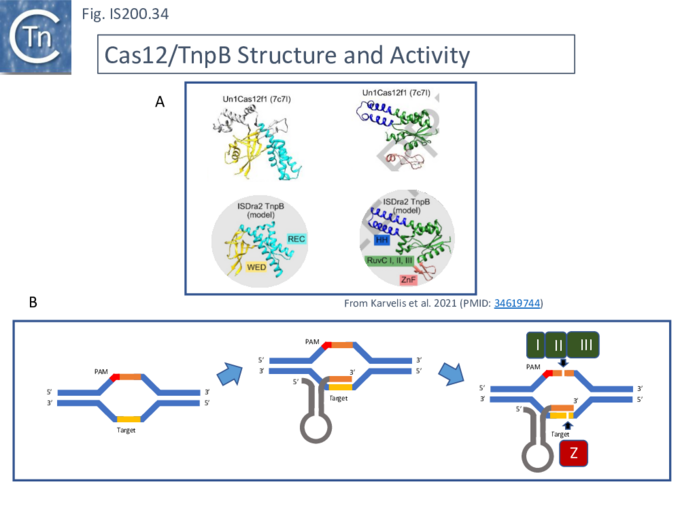
Karvelis, et al.[72] describe the domain structure of TnpB and present evidence that it is related to Cas12, another derivative of the Cas family (Fig. IS200.34 bottom). Like Cas12F, it also carries a RuvC in which the D (I), E (II) and D (III) catalytic residues are split. Again, RuvCI and RuvCII are separated by an R-rich region and RuvCII and RuvCII by a zinc finger with three modules (Fig. IS200.31 bottom). Moreover, the N-terminal region which corresponds to the minimal common structural elements present in Cas12 [72], includes a three helical bundle Rec domain (labelled HTH in an earlier TnpB analysis; Fig. IS200.31 bottom), inserted into a β-barrel domain, referred to as the “Wedge” domain in Cas12. It should be noted that the RuvC domain is used to cleave both DNA strands while the Z domain simply assists this cleavage.
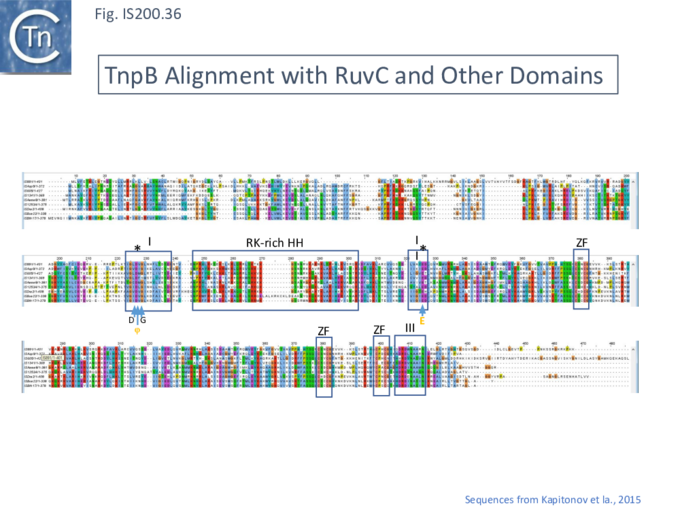
These features can be identified in an alignment of the entire TnpB library (349 examples from ISfinder; November 2021) (Fig. IS200.35 i, ii and iii) and in TnpB sequences provided by Kapitonov et a.,[42] (Fig. IS200.36).
The relationship between Cas12 and TnpB has strong support from structural modelling [72]: for example Un1Cas12f1 (Cas14a) from an uncultured archeon [78], which functions as an asymmetric dimer and represents a minimal domain organization of the Cas12 group [72]. However, TnpB from ISDra2 (see below) appears to be a monomer [72].
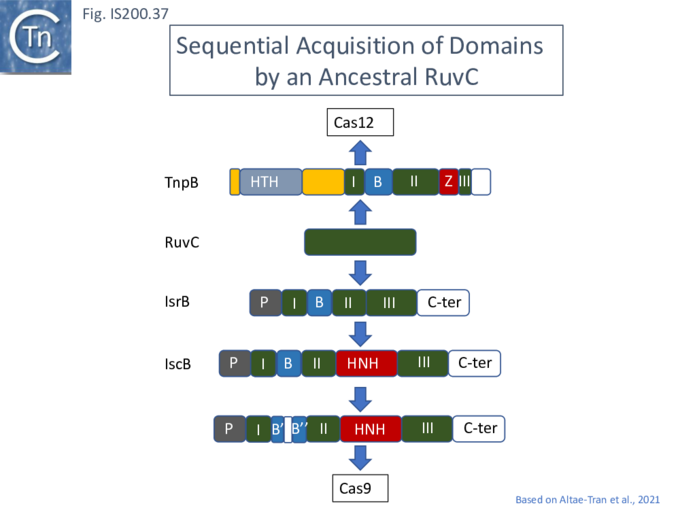
Evolution of TnpB and IscB from an Ancestral RuvC?
In view of the relationship between TnpB, IscB, RuvC and the Cas proteins, the important question of the evolutionary trajectory of these proteins arises. Using various analytic tools, it was concluded that all Cas9 examples identified to date are probably descended from a single IscB derivative ancestor [43]. This contention arose from the observation that the CRISPR-associated IscB derivatives do not form a single clade but are distributed over the IscB phylogenetic tree suggesting that they evolved independently from a single acquisition [43]. Additional IscB derivatives were also identified in this study which led to an evolutionary scenario involving successive acquisition of domains by an ancestral RuvC (Fig. IS200.37). The additional species included a shorter derivative, IsrB, which carried the bridging helix but not the HNH domain and a longer derivative which had acquired a so-called REC domain [43].
TnpB appears to have followed an alternative evolutionary route towards Cas12. In addition, it is thought that TnpB was an ancestor of the eukaryotic Fanzor proteins [80] (see: Fanzor section below) associated with diverse eukaryotic potential transposable elements.
Functional analysis of TnpB and IscB
Clearly, the relationship between TnpB and IscB and Cas12 and Cas9 respectively suggested that TnpB and IscB might function as RNA guided nucleases which may, in some way, be involved in transposition [43][72] and this has been extensively tested.
TnpB functions as an RNA-guided Endonuclease
For TnpB, Karvelis, et al.[72] used ISDra2 as a model system. This has the advantage that its transposition behavior has been well characterized [23][68].
In ISDra2, the 3’ end of the upstream tnpA gene overlaps the 5’ end of tnpB. The authors were unable to efficiently express TnpB as a fusion protein but observed that its yield was significantly increased when in its natural context but in which TnpA had been inactivated by mutation. Although the nature of the mutation is not specified in the article, its behavior could be explained if it were an in-frame deletion or other mutation which does not affect C-terminal translation since it seems likely that expression of TnpB involves translational coupling [81][82] with TnpA suggested by their overlapping reading frames (Fig. IS200.38).
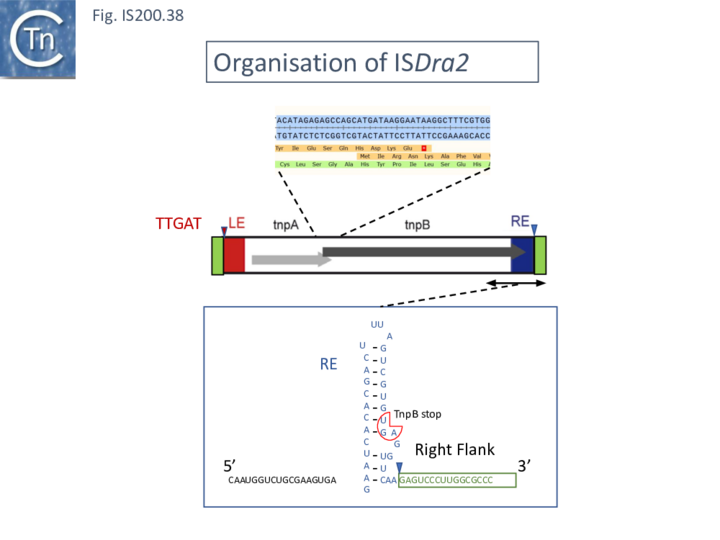
TnpB was found to purify with RNA of approximately 150 nts derived from the IS RE (reRNA). reRNA was complementary to the tnpB 3’ end, RE, and about 16 nt of (host) flanking DNA (Fig. IS200.38). This RNA, with the secondary structure provided by the RE sequence and the 3’ extended flanking DNA is of the expected configuration for relatives of Cas12 (Fig. IS200.36). Previous studies had identified non coding RNA (ncRNA) from the 3’ end of IS1341, a related IS from Halobacterium salinarum NRC-1, called sense overlapping transcripts (sotRNAs) [83].
ncRNAs, sotRNAs and reRNAs
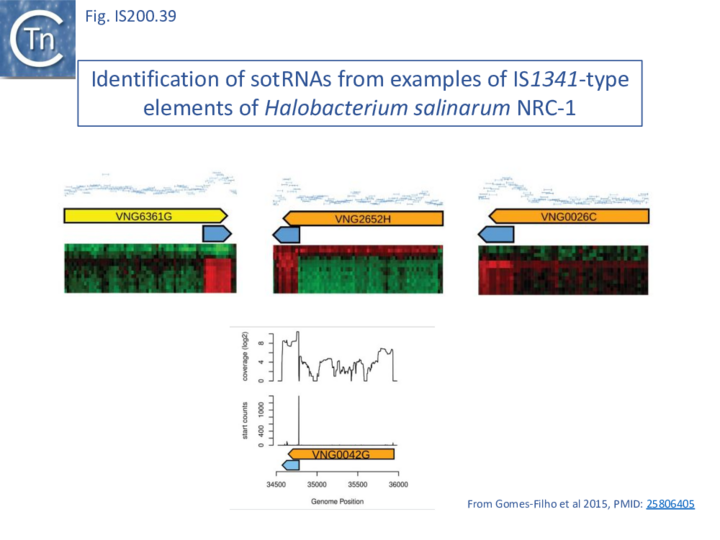
There has been much interest in non-coding RNA (ncRNA) and global searches in Archaea had revealed ncRNA expressed from IS1341 group members which carry only a tnpB gene and are devoid of the TnpA transposase [84][85][86].
During a detailed analysis of ncRNA produced from Halobacterium salinarum NRC-1 [87][88], an ncRNA from the region encompassing the right end of these IS200/IS605 family members was identified. This was called sotRNA (sense overlapping transcript). The authors demonstrated from a publicly available transcriptome compendium [89] that all 10 IS1341 group members in H. salinarum NRC-1 genome express a sotRNA (Fig. IS200.39) and show condition-dependent differential regulation between sotRNAs and their cognate genes. sotRNA started within tnpB at approximately 1100 nt from its initiation codon, had an average size of 218 nt, and ended approximately 74 nt 3’ to the tnpB termination codon. The authors could not distinguish between the hypotheses that sotRNAs are generated by primary transcription or by processing from a full length transcript of the tnp gene (although they were unable to locate any potential promoter).
Such sotRNA transcripts, specific for tnpB genes, had previously been identified by Gomes-Filho et al., [87] in a number of Archaea and Bacteria including S. acidocaldarius, Methanopyrus kandleri, Helicobacter pylori and E. coli K12. There has also been some indication of “transposase-related” sense overlapping transcripts of tnpB-like genes from T. kodakarensis [90] and P. furiosus, [91]. However, that these may represent guide RNAs had not been explicitly considered.
Furthermore, sotRNA included, what the authors called, an RE-like tetraloop resembling the RE DNA loop structure as do sotRNA from P. abyssi and other thermococcal genomes [85].
TnpB: mechanism of action
Karvelis et al.[72] demonstrated that TnpB, purified using a His tag, could cleave DNA. They argued that since the 3’ end of the ISDra2 reRNA corresponds to the DNA target, it would vary according to the position of the IS insertion and the reRNA may (have) serve(d) as a guide RNA. If true, cleavage of the target DNA should occur within the 3’ extension sequence of the flank (the foot of RE in Fig. IS200.38). In this context, it is interesting that the (DNA) structure of the right end was shown to form a base triple which is a characteristic of RNA [21].
To determine whether RNA-guided cleavage occurred , they constructed a system (Fig. IS200.40) using a plasmid supplying TnpB together with an reRNA (Fig. IS200.40 A) which included a 16 (or 20) defined nucleotide flank sequence and was terminated by a specific Hepatitis delta virus ribozyme (HDV; [92]) to produce a defined 3’ RNA end [93]. A lysate from the host strain was then used in cleavage assays of a library of target plasmids each containing a specific defined 16 base pair sequence directly downstream from a 7 bp (7N) randomised sequence (Fig. IS200.40 B). This has previously been used to identify conserved PAM sequences [72] [94]. Specific double strand cleavage products were captured by adapter ligation (details in Karvelis et al.[72] and the sequence of the resulting enriched 7N region was determined. This corresponded to the conserved ISDra2 target pentanucleotide TTGAT (with a higher enrichment for GA) sequence which is essential for IS insertion and abuts LE in the integrated IS. By equivalence to PAM, this sequence was called TAM (Transposon Adjacent Motif) [72] see also [43] (Fig. IS200.40 C).
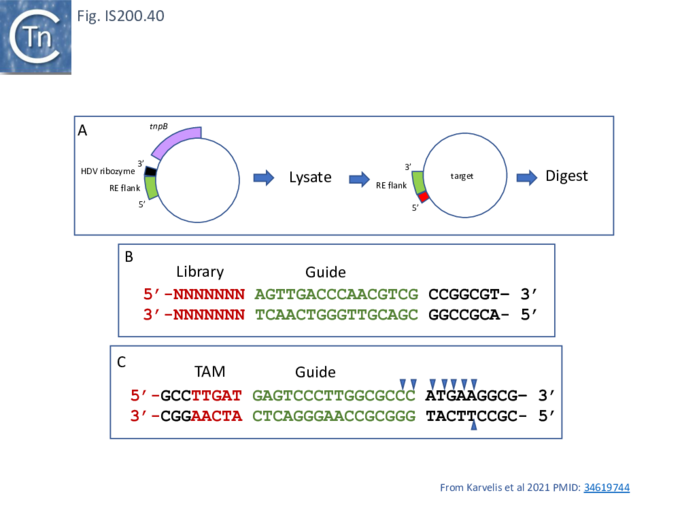
This cleavage specificity was confirmed using purified TnpB-RNP in which the protein and RNA components were produced by separate plasmids and a target plasmid carrying a 3’ flank, a 5’ TTGAT TAM pentanucleotide and a different guide sequence (Fig. IS200.40 C). The results showed a majority of double strand breaks in the supercoiled target plasmid to generate linear plasmids but also a significant level of nicked product. The TnpB-RNP was also active on a linear substrate (i.e. activity does not require supercoiling). In both cases, use of a TnpB D191A mutant, part of the conserved RuvC DED catalytic triad, eliminated the reaction. Robust TnpB-mediated cleavage activity was observed and required both TAM and guide RNA sequences. Further sequence analysis revealed that cleavage occurred distal to the TAM sequence at the guide sequence boundary and was specific for cleavage on the bottom strand but showed some variation on the top strand (Fig. IS200.40). There are some differences however with Cas12. TnpB is a monomer and requires a single copy of reRNA [72].
A similar study by Altae-Tran et al.[43] using purified TnpB from a less well characterised tnpB gene of Alicyclobacillus macrosporangiidus, (TnpBAma), showed that the protein catalysed cleavage of both double- and single-stranded DNA targets in both a TAM-dependent and TAM independent manner. As in the case of TnpBISDra2, A. macrosporangiidus TnpB-associated guide RNA was identified and derived from the 3’ end of the tnpB gene. In this case, the TAM appeared to be the tetranucleotide TCAC.
These studies therefore identify CL (which is outside the transposon but necessary for transposition by interacting with GL Fig. IS200.13) as the TAM.
An explanation of the “inhibitory effect reported for TnpB?
Moreover, in vivo, TnpB expression together with reRNA from one plasmid resulted in loss of a second plasmid carrying the reDNA target (interference), presumably as a result of cleavage at the target site and linearization of the plasmid. This of course may explain the inhibitory effect of TnpB originally observed by Pasternak et al. [68].
A system which functions in Eukaryotes
Additionally, the authors were able to demonstrate that the system functions in eukaryotic cells opening the possibility that it could be suitably modified for gene editing.
RNA Nomenclature, Processing, Structure, Diversity and mode of function
IS605 group guide RNAs have been called both reRNA and ωRNA (OMEGA for obligate mobile element-guided activity). Here, to eliminate confusion, we will use the term re(ω)RNA (or ω (re)RNA) for that from both tnpB and iscB groups although they have different secondary structures and functions.
Generating re(ω)RNA: Processing
The important question of how re(ω)RNA is generated was addressed by Nety et al. [95]. Given that TnpB is thought to be an ancestor of Cas12 [96][97], the ability of Cas12 to process RNA (e.g. [96]) may have originated from analogous functions in TnpB [95]. They demonstrated that a TnpB orthologue from the bacterium, A. macrosporangiidus (AmaTnpB or TnpBAma), has RNA processing TnpBAma activity and can generate an re(ω)RNA.
The purified TnpBAma (either wildtype or a RuvC-II catalytic mutant) was incubated with four different in vitro transcribed RNA substrates (Fig. IS200.41 i and ii) produced from PCR-generated DNA templates: a “random” negative control of 1190 nt (Fig. IS200.41 i1); a 166 nt RNA with the RNA guide very similar to that found to be associated with an TnpBAma orthologue, a potential re(ω)RNA (Fig. IS200.41 i2); a full length tnpB transcript extended to include the guide sequence of 1190 nt (Fig. IS200.41 i3); and the potential re(ω)RNA with a 59 nt 3’ extension of 225 nt (Fig. IS200.41 i4).
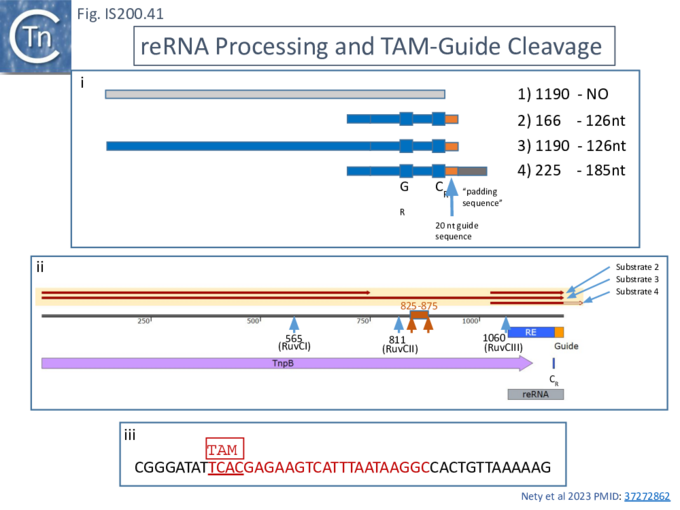
While substrate 1 was refractory to processing, both substrates 2 and 3 generated a 126 nt fragment. Substrate 4 generated a 185 nt fragment suggesting that, while it was processed correctly at the 5’ end, the 3’ extension was not processed. These conclusions were confirmed by RNAseq. All substrates were refractory to the TnpBAma RuvC-II mutant.
DNA cleavage activities were assessed by including a 1221 nt dsDNA substrate containing the TnpBAma TAM (Fig. IS200.41 i). RNA substrates 2, 3 and 4 all catalyzed TnpB-mediated DNA cleavage. These results are consistent with those obtained with TnpBDra2 (see below;[98][99]) showing that only the proximal 12 nt of the guide sequence is sufficient for DNA targeting.
The cleavage activity of the three substrates was not identical. The activity of substrate 3, which carries a substantial 5’ extension, was significantly lower than the other two raising the question of whether the extension may include inhibitory sequences.
To investigate this, RNA samples were prepared with different 3’ deletions (Fig. IS200.41 ii) When these RNA species were included in the cleavage reactions, a region between co-ordinates 825 and 875 which shows extensive complementarity to the re(ω)RNA scaffold was observed to be responsible for the inhibitory effect.
This suggests a cis-regulatory mechanism engaged in controlling re(ω)RNA activity [95].
Using ISDra2 [23], Nakagawa et al.,[98] observed that, although TnpB was co-expressed with a 247 nt re(ω)RNA in their purification system, it remained bound to only 100-160 nt of the RNA even in a denaturing gel. Further analysis revealed that the RNA was rapidly degraded in the absence of TnpBDra2 but, in its presence, three different RNAs of approximately 220, 160 and 130 nt were observed, the latter two included the guide sequence at the 3’ end. Very little of the 200nt species was observed in the purified RNP, suggesting degradation, but LC–MS analyses suggested that the 160nt species was cleaved between co-ordinates −150 and −149 or −138 and −137 by TnpB and/or endogenous RNases. They also provide evidence that the ~130-nt RNA is cleaved between −117U and −116G (Fig. IS200.41 ii).
Furthermore, Sasnauskas et al., [99], observed that an re(ω)RNA from between co-ordinates -130 and + 16 was active in DNA cleavage. Nakagawa et al.,[98] also found that truncation of the 5′ region of the re(ω)RNA (−231G to −117U) had no effect on TnpB-mediated DNA cleavage.
Thus re(ω)RNA of ISDra2 also appears to be processed at its 5′ end, and at least a 130 nt fragment including the 3’ guide are stably bound to the TnpB protein.
Structure of TnpB-reRNA in association with DNA
Two studies addressed how TnpB interacts with its DNA template [98] [99] both used TnpBDra2. (Fig. IS200.42). and an re(ω)RNA which included nucleotides -130 to + 16 of the right end (Fig. IS200.42 ii) [99]. Nakagawa et al., [98] used a substrate which was slightly extended in the 5' direction. Both sets of results were essentially the same.
The RNP structure and the ternary structure with the target sequence TnpB could be divided into two “lobes” [98][99]: an N-Terminal lobe (Recognition or Rec) comprising the wedge (WED) and REC domains and a nuclease lobe (Nuc) (insert in Fig. IS200.42 iii) in which the three individual RuvC domains adopt an RNase H fold including D191 (RuvC I), E278 (RuvC II) and D361 (RuvC III).
The results showed that in the RNP complex (Fig. IS200.42 iii left), the principal interactions are with the RuvC and WED domains whereas in the ternary structure with target DNA (Fig. IS200.42 iii right), not only does WED interact with TAM but the RecA domain intervenes around the branch point and the RuvC domain interacts extensively with the target-guide RNA hybrid helix. Note that the CR (TAM) sequence which interacts with GR as DNA during TnpA-mediated transposition ( Fig. IS200.42 i) also forms a short interaction with a sequence upstream which is identical to GR (Fig. IS200.42 ii) to generate a pseudoknot. The scaffold core is formed by the RNA triplex region delimited by the pseudoknot while stem 1 and stem 2 protrude in opposite directions (Fig. IS200.42 iii).
All five TAM positions (Fig. IS200.42 iii right) are recognized directly by the WED domain and substitutions at any TAM position eliminates both target DNA binding and cleavage [99].
On the other hand, substitutions in the guide sequence do not prevent TnpB binding but prevent cleavage. The re(ω)RNA–target DNA heteroduplex (Fig. IS200.42 iii right) is accommodated within a central channel formed by the WED, REC and RuvC domains [98][99].
The authors conclude from the structural results that, for cleavage, the system senses formation of a (perfect) B-form RNA-DNA hybrid without any mismatches because of the effect of guide substitutions and that TnpB requires a 12–16-bp long target perfect DNA-guide RNA heteroduplex to initiate DNA cleavage.
Additional information concerning activity was provided in a study principally exploring diversity in this system (see: Exploring and defining TAM sequences).
Xlang et al [36] analyzed re(ω)RNA activity requirements of ISDra2 and three additional IS: ISTfu1, ISDge10 and ISAba30. In these experiments, the 3’ re(ω)RNA scaffold end was defined as the RE tip (Fig. IS200.44).
Activity was exquisitely sensitive to the integrity of CR. Deletion or mutation of all but the 3’ terminal CR base pair significantly reduced activity.
Additionally, the length of the guide sequence was important as was its sequence matches with the target. Optimal editing efficiency occurred with guide sequences between 16 and 20 nucleotides and subsequently decreased with increasing length but was observed to vary somewhat between the three IS (Fig. IS200.42 ii).
Similarly, introduction of single and double base pair transversions into the target, especially in the TAM proximal region approximately up to base pair 12, severely reduced or eliminated activity (Fig. IS200.42 ii) with some variation between the different IS.
This is similar to results obtained with Cas9 and Cas12 systems themselves [100][101]. Finally, variation in 5’ length showed that shortest active scaffolds were 120–140 nt long and lengths of 300 nts were active.
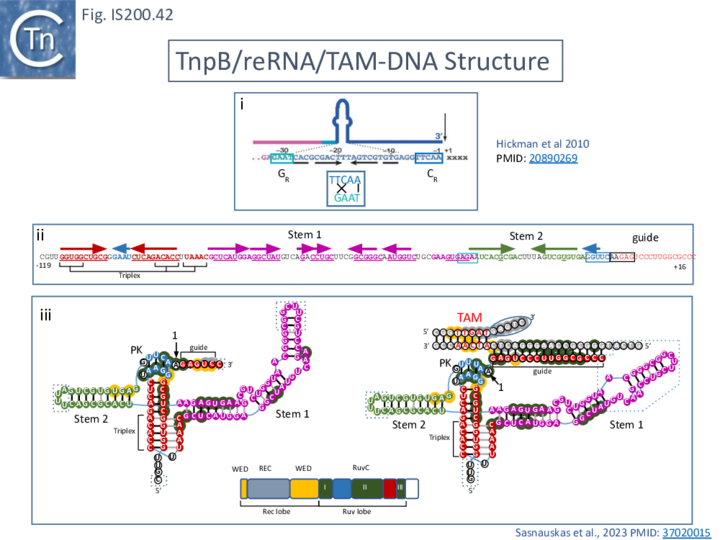
For TnpBDra2, the C-terminal domain (residues 376 to 408; Fig. IS200.42 bottom insert) has relatively low sequence similarity among TnpB proteins and is disordered in the structures. The C-terminal truncation mutant (Δ376 to 408; ΔCTD) is efficient in target DNA cleavage but exhibits somewhat reduced protein stability. Thus the CTD is not required for RNA-guided target DNA cleavage.
TnpB-re(ω)RNA: Diversity and Activity
In view of the minimal size of the TnpB family guide endonucleases, they may prove useful for targeting applied for biotechnological purposes. It is therefore of importance to determine the extent of their diversity and inherent activities. It had been reported that the TnpB family is an order of magnitude more diverse than the IscB family and an HMMER search of prokaryotic genomes identified >106 tnpB loci [43].
At least two studies [36][95] have addressed this question in some detail.
Exploring and defining TAM sequences
To further explore TnpB diversity tnpB DNA sequences of the 107 IS605 subgroup ISfinder entries (Fig. IS200.4B) were more extensively analyzed [36]) with a view to uncovering differences in activities and identifying highly active members. This analysis did not include the 244 IS1341 members which are flanked by typical IS200-IS605 family secondary structures but carry only a TnpB gene.
Firstly, the IS605 subgroup members were used as a seed to search the non-redundant NCBI nucleotide sequence database. Full length copies were extracted and their flanking sequences were examined to eliminate identical insertion events.
To confirm the ISfinder validation, the right end of each multicopy IS was aligned and the tetranucleotide which forms CR and undergoes special base pairing with the tetranucleotide guide sequence (GR) within RE (Fig. IS200.13) was identified, while the single copy IS were examined and compared to their ISfinder annotations. Additionally, the integrity of tnpB was confirmed. This is important because it has been observed that in IS containing tnpA and tnpB, tnpB is often decayed (see He et al., [102]).
It should be noted that these procedures are always undertaken as a matter of course before any IS200/IS605 family entry is made in ISfinder.
The collection was arranged into 64 bins using a 90% identity threshold and these were named after the IS with the highest copy number in each group (Fig. IS200.43). Many of these groups consisted of only single example although several included a few additional examples.
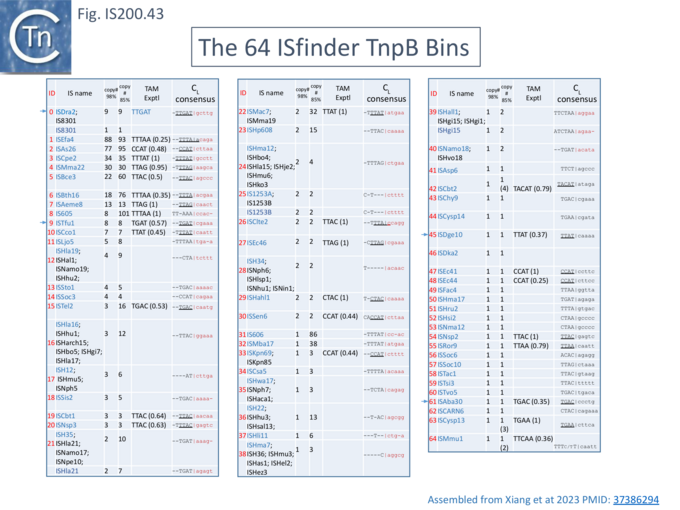
To examine how the sequence identities between CL and TAM (Fig. IS200.44) correlate over the range of IS605 group members distributed over the 64 TnpB bins (Fig. IS200.43), activities were tested separately for each of the 64 using a 2 plasmid, TAM depletion assay (Fig. IS200.44 ii) [36].
One plasmid included ~200 nucleotides of the 3’ IS ends including a 20nt abutting “guide” sequence cloned downstream of a tnpB gene which, when expressed together (Fig. IS200.44 ii), are capable of forming the re(ω)RNA complex. The second plasmid consisted of a library with five randomized base pairs (N5) located 5’ to a target sequence recognized by the guide sequence, an assay similar to that used by Karvelis et al., [72] (Fig. IS200.40). Both plasmids were introduced concomitantly into a host cell. Those that carry an N5 sequence susceptible to the corresponding re(ω)RNA complex will be depleted and underrepresented in the plasmid population (reduced level of KmR colonies in the population).
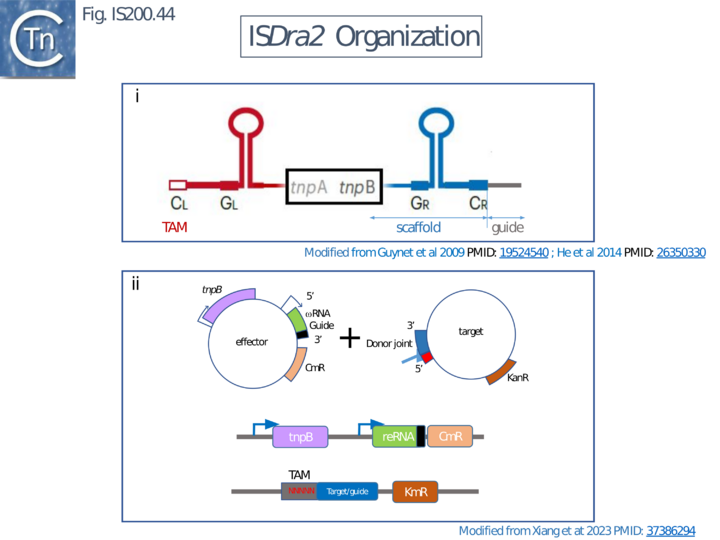
The corresponding TAM sequences (Fig. IS200.43) showed a remarkable identity to the CL sequences with very few variations. For these variants, the authors propose alternative base pairings which would need to be confirmed experimentally.
Further analysis based on a tree generated from TnpB alignments such as those shown in Fig. IS200.35, revealed, perhaps not unexpectedly, that TAM sequences were more similar between closely related IS.
The relative activities of the TAM sequences in each case were then assessed in E. coli using a similar plasmid system to that of Fig. IS200.44, but in which the N5 sequence was substituted for the proposed TAM.
A high proportion (25/64) of these TAM/TnpB derivatives were found to be active.
Sequence requirements of the re(ω)RNA
To explore re(ω)RNA sequence requirements in greater detail, three IS systems, ISTfu1, ISDge10 and ISAba30, in addition to ISDra2, were analyzed in for their guide RNA functions [36].
The relatively small TnpB protein had been demonstrated to function in gene targeting in human cells [72]. Since the interest of Xiang et at [36] was to optimize TnpB as a targeting tool in human cells, the assay was designed for use by transfection into the HEK293T human cell line It used a system in which an out of frame downstream GFP gene was reframed only when the TnpB nuclease could act on its target and the DNA break was repaired by non-homologous joining (Fig. IS200.45 i).
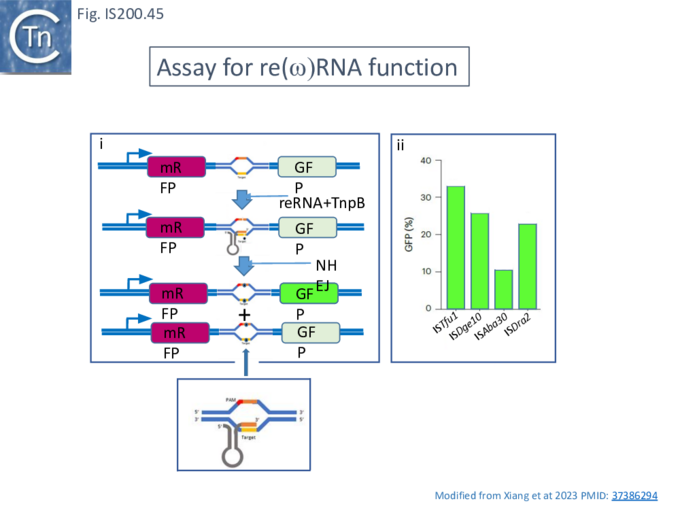
When this reporter plasmid and a TnpB/ re(ω)RNA plasmid were co-transfected, all four TnpB systems were shown to function, yielding 10% to 34% of GFP transfected cells (Fig. IS200.45 ii). They each generated short, deletions of various lengths, some of which lead to placing the GFP gene in phase yielding GFP+ cells in the population. The overall organization of the IS including TAM, scaffold and guide sequence is shown in Fig. IS200.46 i.
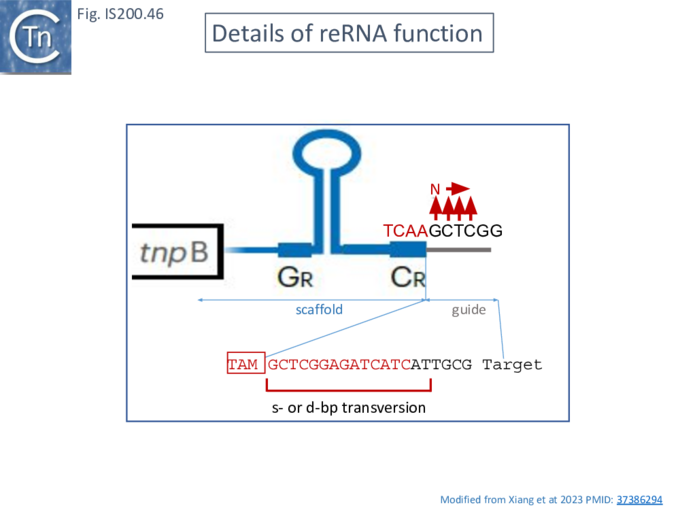
Severely decreased activity in re(ω)RNA guide activity was observed with mutation of either CR or the four proximal nucleotides (Fig. IS200.46) and in the target site with single or double transversion in the TAM proximal region.
It should be noted that where assays were carried out following transfection of human HEK293T cells and it is possible that the results may vary in the appropriate bacterial hosts.
Exploring and defining TAM sequences in a library extracted from NCBI
In a second study to investigate whether the re(ω)RNAs were present across the widely diverse TnpB systems [43], Nety et al.,[95] constructed a TnpB sequence library, extracted from data from NCBI, which included those associated with Y1 (HUH; IS200-IS605 family), serine (IS607 family) transposases or “non-mobile” orthologues. This generated 5 clades [95]; background in Fig. IS200.47). The clades follow the configuration of the RuvC catalytic motif (Fig. IS200.47) (RuvC-III DRDXN, typical; RuvC-III NADXN, derived) or “catalytic rearrangements (RuvC-II (RII-r3 and 5) or RuvC-III (RIII-r4) domain) [103] (Fig. IS200.47).
The authors chose 59 TnpB orthologs covering the diversity (background to Fig. IS200.47; [95] and varying in length between 353 to 550 aa. The TnpB-re(ω)RNA-encoding loci including a suitable promoter were expressed in an in vitro transcription/translation (IVTT) system and the 5’ ends were determined by RACE from the 3’ re(ω)RNA end lacking the guide sequence.
This identified 30/59 orthologs with a defined 5’ end and lengths of between 79 and 466 nt. TnpBAma generated a 106 nt scaffold, and is thus identical in processing as was found in the experiments of Fig. IS200.41. Some orthologs, such as TnpBDra2 showed multiple 5’ ends, consistent with previous observations suggesting either incomplete or promiscuous RNase activity [72][98].
A screen for DNA nuclease activities of the IVTT-produced re(ω)RNAs revealed that 27/59 were active. They also defined the TAM sequences revealing only limited diversity of these sequences as was also found for the ISfinder collection [36]. The assay was validated by confirming both the TnpBAma (TCAC) and TnpBDra2 (TTGAT) TAM sequences.
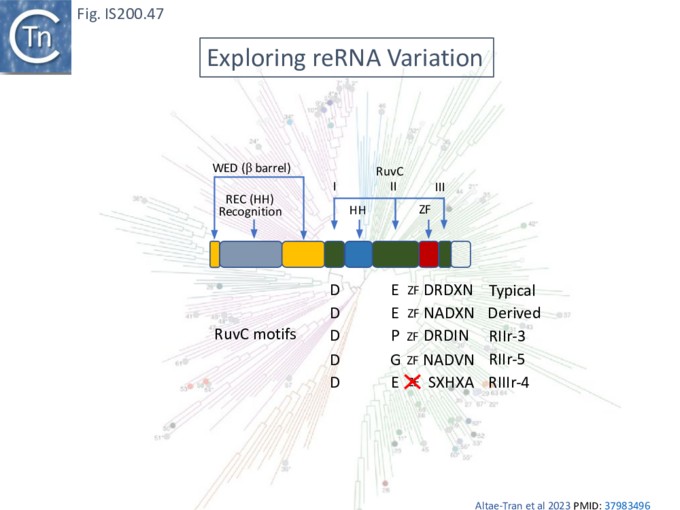
re(ω)RNA and tnpB Co-evolution
It was noted that ISDra2 re(ω)RNA includes the 3’ segment of tnpB (residues 335 to 408 and −231G to −10U) which suggests that TnpB and the guide sequence system might have co-evolved [99]. However, although re(ω)RNA expression and processing may require co-expression with the TnpB protein, Nakagawa et al., [98] suggest that co-evolution might be less constrained than previously predicted because, they argue, that functionally essential gene regions and those of re(ω)RNA do not overlap significantly: the structures imply that the TnpB C-terminus (residues 376 to 408 overlapping with −109G to −10U) is not involved in DNA cleavage, and the 5′ re(ω)RNA terminus (−231G to −117T, overlapping with residues 336 to 373) is not required for target DNA cleavage.
The question of co-evolution is complex since it must also take into account the constraints imposed by the mechanism(s) involved in the DNA transposition process: the TAM sequence which abuts the left IS end (LE) also serves as a sequence required for cleavage and insertion at the left end CL and that CL interacts in a complex way with a partially complementary sequence, GL, located at the foot of a stem loop (DNA) structure recognised by the TnpA transposase (see He et al., [102]). Moreover, changing the GL sequence leads to a change in the specificity of insertion – i.e. changes the CL sequence [48]. More importantly, the CR sequence which is an integral part of the IS, plays a central role in both the RNA guide and TnpA-mediated DNA cleavage reactions and interacts both with a sequence at the foot of a secondary structure at the right end (RE), GR. and, in the re(ω)RNA where it forms part of a pseudoknot (Fig. IS200.42) [98] [99].
IscB, like TnpB, is also an RNA-guided Endonuclease
Altae-Tran et al.,[43] also examined a very large number of rather disperse IscB systems for their endonuclease properties, their association with RNA and their capacity as RNA guide proteins. Initial studies concerned a CRISPR associated IscB (marked in the article as Delaware Bay acquatic sample), which when purified from a heterologous Escherichia coli host was associated with an RNA localised directly upstream of iscB which generated a signal in a PAM (TAM) “discovery” assay and was able to generate cleavage products in vitro with the appropriate target.
An alignment of over 500 (non-redundant) iscB genes revealed an upstream region of conserved sequence of about 300 bp which terminated at what the authors state is an IS200/IS605-like end. One specific example examined, present in the host K. racemifer genome in nearly 50 copies, was associated with non-coding RNA species in most cases, which they called ΩRNA, with significant secondary structure potential. An example of K. racemifer IscB was investigated in vitro using a plasmid substrate and shown to: use a target adjacent pentanucleotide TAM, ATAAA; and observed that by changing the complementary RNA extension (guide),cleavage was reprogrammable.
To further characterize IscB, the TAM sequences of 57 examples from a collection of 86 genes from a phylogenetically diverse set of bacteria could be determined; of those 57, 5 were reconstituted with their omega RNA and found to be active in target cleavage; and one, AwaIscB or IscBAwa) from Allochromatium warmingii, was chosen for further study.
Biochemically, IscBAwa could cleave double strand DNA in a magnesium dependent reprogrammable way with a temperature optimum of 35-40°C and with RNA guide lengths of between 15 and 45 nts. A mutation of the RuvC E residue eliminated cleavage of the non-target strand while mutation of an H residues in the HNH motif eliminated cleavage of the target strand (as expected for a Cas9-related enzyme; Fig. IS200.32). Mutation of both residues eliminated cleavage altogether. Also, like Cas9, cleavage was: TAM (PAM) proximal (3 nts from TAM for the target and 8 or 12 nts for the non-target strands); the RNP protected DNA from ExoIII digestion 19 nts upstream of the TAM on the target and 6 downstream on the non-target (Fig. IS200.32); and truncation of the newly identified N-terminal PLMP domain (named after a cluster of conserved amino acids; Fig. IS200.48 top) eliminated activity.
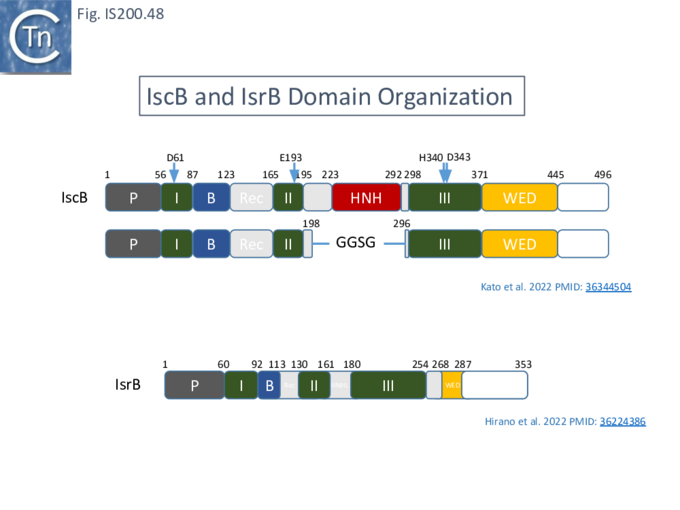
The Structure of IscB–ωRNA ribonucleoprotein complex and the ternary complex containing target DNA.
IscB associates with a 200-400nt ωRNA, significantly longer than the 100nt guide RNA of its probable offspring, Cas9 [43]. IscB are much smaller than Cas9 and lack the α-helical nucleic-acid recognition domain but share the RuvC and HNH endonuclease domains (Fig. IS200.48).
Kato et al., [104] used an IscB protein derived from the human gut metagenome (IscBOgeu) as a model while Hirano et al., [105] used an IrsB (IsrBDt) from Desulfovirgula thermocuniculi. IrsB are related to IscB but lack the HNH nuclease domain (Fig. IS200.48). Note that this is a more detailed description of the domain structure than shown in Fig. IS200.37. A detailed study by Meer et al., [41] found that the IscB and IrsB formed clearly separate groups on a phylogenetic tree.
For the structural cryo-em studies, a catalytically inactivated IscBOgeu E193A (RuvC)/H247A (HNH) derivative was used. In the IscBOgeu structure, the catalytic D61 (RuvC I), E193 (RuvC II), H340, and D343 (RuvC III) and a divalent Mg2+ ion (Fig. IS200.48) are configured similarly to those in Cas9 although the structure lacked the HNH domain.
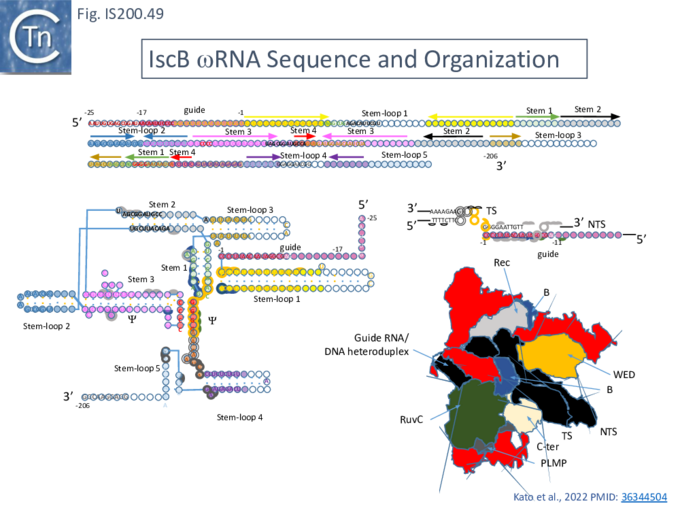
The ωRNA structure is complex (Fig. IS200.49) comprising a 27 nt guide sequence and a 206 nt scaffold with 5 stem loops, 4 stems and a linker. The guide adaptor, stem-loop 1 (yellow), connects the guide segment (dark red) and stem 1 (green; which the authors call the “nexus” stem widely conserved in the tracrRNA of Cas9s; [106]). Stem 1, stem 2 (grey; the central stem), and stem-loop 3 (brown) form a three-way junction. Like TnpB ωRNA, IscBOgeu ωRNA also includes a pseudoknot (??). Stem loop 2 (blue) stacks with the nexus pseudoknot hairpin (pink) which in turn interacts with the pseudoknot stem 4 (red).
The cognate ωRNA and IscBOgeu E193/H247 were expressed in E.coli, the IscB-ωRNA complex purified and the ternary complex assembled by mixing with target DNA. However, to improve resolution, it was found necessary to delete the HNH domain (residues 199 – 295) (Fig. IS200.48), which is flexible in Cas9 [107][108]. The complex, composed of an IscB monomer and a single ωRNA was formed using the deletion derivative IscBω, an ωRNA of 233 nt including a 27 nt guide sequence and a partially double strand DNA target (Fig. IS200.49 right).
In the ternary complex IscB ωRNA guide sequence forms a 14 bp heteroduplex with the target DNA (Fig. IS200.49 middle right) and is recognized by IscB in a sequence-specific fashion using the short Rec region (Fig. IS200.48) shown in grey in Fig. IS200.49 middle right. A simplified cartoon is shown in Fig. IS200.49 bottom right. This is somewhat different from Cas9 which form a 20 bp heteroduplex with a much larger Rec domain. TAM is recognized by the CT domain and mismatches at positions 15 and 16 are tolerated for cleavage. The differences in a full complex with the HNH domain and with the ωHNH IscB derivative is shown in Fig. IS200.50.
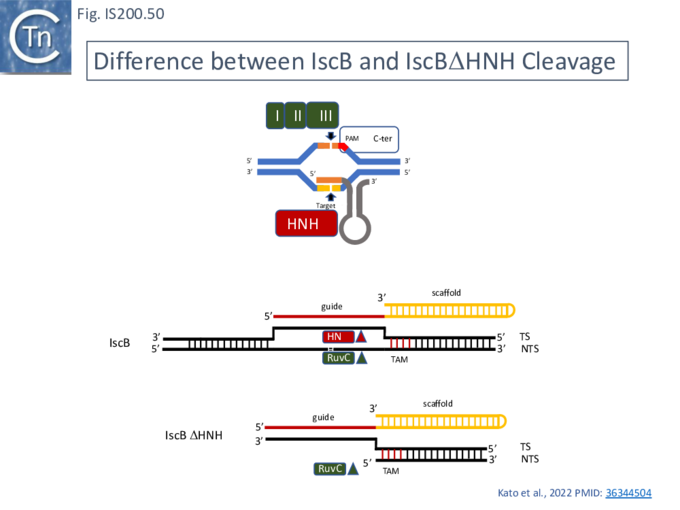
The Structure of IsrB–ωRNA ribonucleoprotein complex and the ternary complex containing target DNA
IsrB is short, about 350 amino acids and lacking an HNH domain (Fig. IS200.51) (therefore equivalent to the ΔHNH IscB derivative). It is associated with a long RNA guide of ~300-nt which guides IsrB to nick the non-target strand (NTS) of double-stranded (ds) DNA (see Fig. IS200.51 top) containing a 5′-NTGA-3′ TAM [43].
The Desulfovirgula thermocuniculi IsrB (IsrBDt) ωRNA (284 nt) is longer than that of IscBOgeu, and includes a 20 nt guide segment which forms a heteroduplex with the target DNA [105]. Like IscBOgeu, IsrBDt ωRNA is structurally complex including eight stem loops and four stems (Fig. IS200.51 middle). The structure includes 2 pseudoknots: one defined by two of the stem-loops (2 and 5, red boxes (Fig. IS200.51 middle) and the other the “nexus” pseudoknot (blue boxes).
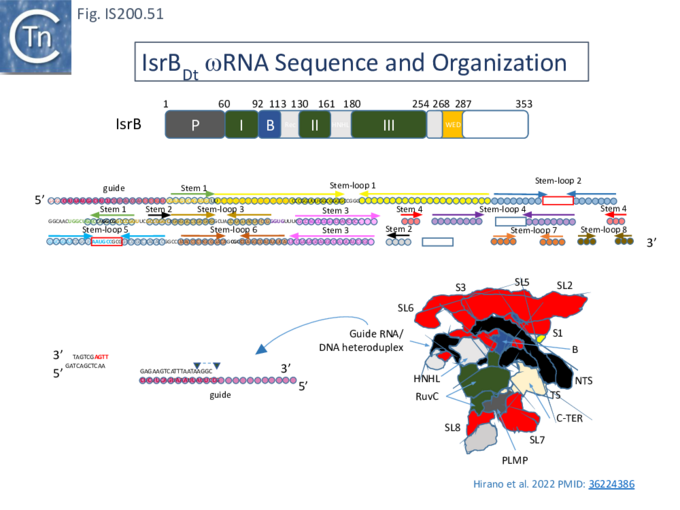
IsrBDt recognizes the TTGA TAM in the NTS by both hydrogen bonds and van der Waals interactions and cleavage occurred 8–11 nt upstream of TAM, further than the 2–5 nt of Cas9. TAM recognition was more specific at 60 °C for this thermophilic enzyme than at lower temperatures where NTGA was recognized [43].
IsrB diversity of structure and ωRNA architecture
As in numerous publications in this field, Hirano et al., [105], explored IsrB diversity and ωRNA ternary structure. They identified five orthologues and their cognate ωRNAs from: Crocosphaera watsonii (IsrBCw); Dolichospermum sp. (IsrBDs); Calditerricola satsumensis (IsrBCs); Burkholderiales bacterium (IsrBBb); and a viral metagenome assembly (IsrBK2). A standard TAM identification assay (such as that shown in Fig. IS200.40) indicated that IsrBBb recognizes NTGG while IsrBCw, IsrBCs, IsrBDs and IsrBK2 recognize NTG. All were active in an in vitro reconstituted IsrB-ωRNA RNPpromoted nicking of dsDNA substrates.
ωRNAs of the five orthologues and IsrBDs retain the core domain composition: four stems (S1–4) and five stem loops (SL1/2/4/5/7) (Fig. IS200.51 middle). Inspection of the ωRNAs showed some significant architectural differences, however: For example, in a group, including IsrBCs, IsrBK2 and IsrBBb, SL2 and SL4 form pseudoknots, and SL5 and the intermediate region between S2 and SL7 form pseudoknots while in a second group, including IsrBDt, IsrBCw and IsrBDs, SL2 and SL5 form pseudoknots, and SL4 and the intermediate region between S2 and SL7 form pseudoknots.
The IS1341 Conundrum: how do derivatives without their transposase transpose?
It had been noted that there are a large number of IS200-IS605 relatives which carry only the TnpB gene flanked by typical S200-IS605 family secondary structures [102] in a number of bacteria including the thermophilic Geobacillus and the cyanobacterium Anabaena. These are grouped into the subfamily, IS1341 with nearly 250 entries in ISfinder (December 2023) and were not included in the study of TnpB/TAM diversity of Xlang et al., [36]. Since the multiple IS891 copies such as those found in Anabaena imply that IS1341 group members are mobile, the question arises as to how their mobility might be accomplished. One possibility is that this is assured by a tnpA copy in the cell or that tnpB itself is involved.
IS1341 Group Diversity: Mining the NCBI NR database
The entries in ISfinder do not necessarily reflect the abundance of the different IS200-IS605 derivatives in the prokaryotic kingdom and Meer et al., [41] mined the NCBI NR database for tnpB and iscB homologues and extracted their flanking genomic regions to provide some perspective of the proportion of tnpB genes associated with IS1341 group members.
They found that only 25% of tnpB were associated with a tnpA copy. Note that nearly half IS200/IS605 members in ISfinder do not carry the tnpA gene.
Moreover, in the same analysis, iscB genes were much less abundant than tnpB and only 1.5% of these were associated with tnpA. Additionally, 8% of the tnpB collection were associated with a serine recombinase and are therefore probably members of the IS607 family while none of the iscB genes were found associated with this type of enzyme.
Both IscB and TnpB use transposon-encoded RNAs: For the IscB copies, a conserved intergenic region upstream of iscB that was bounded by the transposon RE was observed , which bore marked similarity to a non-coding RNA termed HEARO (HNH Endonuclease-Associated RNA and ORF; [109]) and those encoded downstream of tnpB have of course been known for some time, initially in Halobacteria (ncRNAs, sotRNAs and reRNAs;[87][88]).
Conserved secondary structure motifs
Covariation in the collection of tnpB and iscB re(ω)RNAs was analyzed separately to highlight the conserved secondary structure motifs (Fig. IS200.52) which straddle the IS ends and flanking DNA. This means that the (external) guide sequences (NNN… in Fig. IS200.52 i and ii) change with each transposition event into another target.
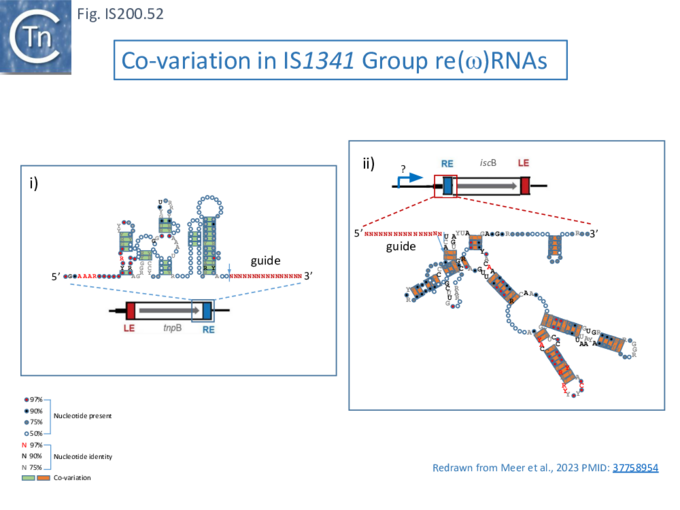
IS1341 group orientation suggests iscB re(ω)RNA but not tnpB re(ω)RNA is expressed in transcriptionally active environments.
A strong correlation was noted in orientation of between upstream genes and istB copies but not for tnpB copies. This is an important observation since it suggests that the re(ω)RNA of iscB must be expressed from an outside promoter towards iscB (Fig. IS200.52 ii) thus favoring production when inserted into transcriptionally active regions, while that of tnpB as shown previously (Generating re(ω)RNA: Processing) is expressed by processing from the tnpB transcript (Fig. IS200.52 i).
IS1341 Group Function
More detailed studies focused on tnpB- and iscB-carrying elements from G. stearothermophilus representing 1% of the genome [41]. These could be divided into 5 families (ISGst2-6) based on tnpB, RE and LE sequences and have quite similar RE and LE boundaries and all exhibited clade-specific CL (=TAM) and CR (=TEM) (e.g. Fig. IS200.44 i; Fig. IS200.53) with clade-specific co-varying mutations between both the TAM and TEM sequences and associated DNA guide sequences (e.g. Fig. IS200.44 i). Evidence from RNA-seq also showed that re(ω)RNA was expressed from multiple copies of these transposase-less IS (i.e. at different genomic positions and with different guide sequences). Derivatives lacking any protein-coding gene PATES (Palindrome-Associated Transposable Elements) [110] were also identified.
Does a Resident TnpA copy Drive IS1341 group Transposition?
Importantly, the authors also identified a tnpA gene in the G. stearothermophilus genome within ISGst2 which might serve to drive transposition of these IS. To asses this, a plasmid-based excision system was used which included a cloned copy of tnpA, (TnpAGst) to catalyze excision of a mini ISGst2 (Fig. IS200.53).
Excision in vivo as monitored by a PCR reaction appeared robust not only with ISGst2 but ISGst3, ISGst4 and ISGst5 all gave robust excision reactions (while that of ISGst6 was weak) and all generated the expected donor junction sequence after excision. The reaction was dependent on an active TnpA catalytic site. However, a substrate derived from IS608 was inactive presumably because it lacks an upstream domain (Fig. IS200.30). Interestingly, excision occurred when the mini-IS was present on the leading or lagging strand template but required both LE and RE. Mutation of the TAM (CL sequence) or the guide sequence (GL) reduced or eliminated activity but compensatory mutations which should restore the CL/GL interactions [48] restored some excision activity (Fig. IS200.53). Perhaps surprisingly, mutation of TEM (GR) did not eliminate excision since the system was able to select an alternative wildtype TEM sequence downstream to create an alternative IS end. It seems possible that, since LE is involved in both excision and targeted insertion, its correct interactions between GL and CL may be more stringent for activity.
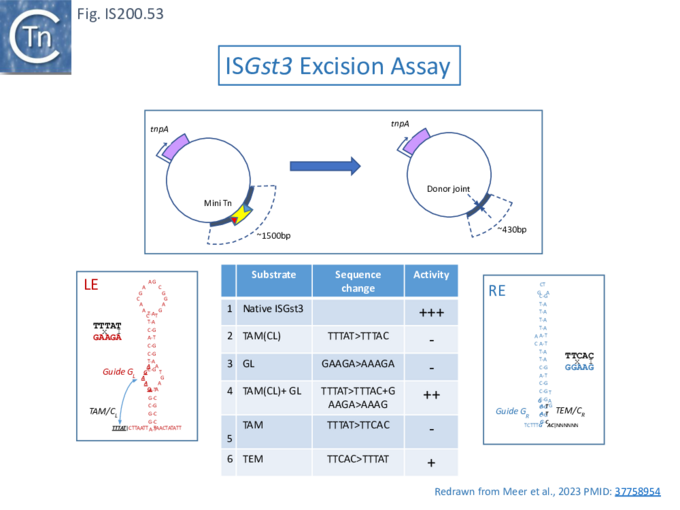
The authors also determined that, although not in single chromosome copy, the cloned tnpA could drive transposition at similar frequencies whether in cis or in trans thus reinforcing the idea that a single tnpA gene could drive transposition of IS1341 group members in the same cell as measured in a mating out assay (TnpBGst and IscBGst proteins are active RNA-guided Nucleases below).
However, it is puzzling that IS1341-like elements of both the TnpB and IscB type have proliferated extensively compared to the “full length” IS. This raises the question of the way in which these genetic objects arose and their function in the cell. It would be interesting to undertake a reconstruction experiment using a single chromosomally located TnpA copy together with a single IS1341 group IS to follow the kinetics of transposition over the long term to determine whether, as seems to be the case with G. stearothermophilus, an accumulation of these pared-down IS occurs.
TnpBGst and IscBGst proteins are active RNA-guided Nucleases
The activity of the ISGst encoded proteins, TnpBGst and IscBGst, was also explored [41] using a two-plasmid interference assay: the effector plasmid tagged with Spectinomycin resistance and which expresses ωRNA with a fused guide sequence together with a cloned copy of TnpB or IscB and a target plasmid tagged with kanamycin resistance which carried the TAM sequence and DNA flanking RE (Fig. IS200.54 A).
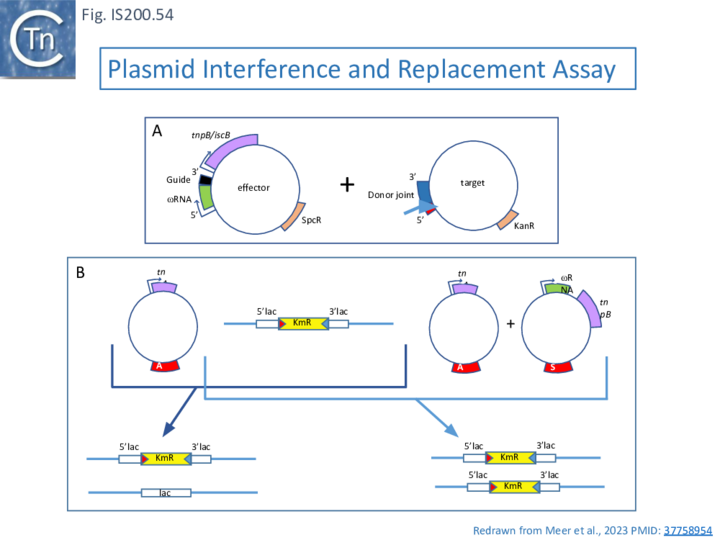
The target sequences were verified as the donor joint generated by IS excision. Successful targeting results in plasmid loss (loss of kanamycin resistance-carrying plasmid due to double strand breakage and loss of cell viability under selective conditions. IscBGst (IscB, ISGst6) and three distinct TnpBGst (TnpB1, ISGst2; TnpB2, ISGst3; TnpB3, ISGst4; and TnpB4, ISGst5) homologues were highly active for RNA-guided DNA cleavage of their native donor joints.
TnpB of ISDra2 was highly active in this type of assay but that of IS608 was inactive presumably because TnpBIS608 lacks the N-terminal HTH domain (Fig. IS200.30)
Additionally, they cloned the native ISGst3 (TnpB3) and demonstrated that its TnpB2–ωRNA robustly cleaved not only the target plasmid but also a plasmid devoid of the donor joint. When a tnpA gene was inserted upstream of tnpB in ISGst3, it proved competent for transposition in a mating out assay at levels ‘in cis’ comparable to when “in trans”.
Binding specificity was then examined using ChIP–seq and catalytically dead IscB and TnpB programmed with lacZ-specific ωRNAs (i.e. ωRNAs in which the guide sequence was complementary to a short sequence in the lac gene) to map chromosomal binding sites of nuclease-dead. These results revealed both the “on-target” (lac) site but also numerous off-target sites, indicating that the Cas12 (TnpB) and Cas9 (IscB) evolutionary “parents” show less dependence on RNA–DNA complementarity for stable DNA binding than their Cas12 and Cas9 descendants. The results also suggested that they might show a higher reliance on a more extensive TAM motif.
TnpB is Required for Replacement of the Deleted IS Copy
“Peel and Paste” transposition [102] implies that the transposition would result in loss of the IS from the lagging strand of the “donor’ replication fork (Fig. IS200.22). Excision of the IS creates a perfect donor joint. The TnpBY/ωRNA endonuclease/guide RNA system could provide a solution to this by “intercepting” the donor joint and retargeting it, recopying the remaining IS copy back into the empty site [72]. The model, developed from data obtained with Deinococcus radiodurans ISDra2, was elegantly confirmed by Meers et al., [41] using a ISGst-based IS.
Using a mini-IS tagged with kanamycin resistance and inserted into the lacZ gene in the E.coli, chromosome, excision could be measured by the appearance of lac+ colonies while transposition could be measured by retention of kanamycin resistance in the presence of non-targeting or lac targeting ωRNA, TnpA (wild type or catalytic mutant and or TnpB (wildtype or catalytic mutant) (Fig. IS200.54 B). The results showed that: a large fraction of colonies were lac+ in the presence of wild-type but not mutant TnpA; this was reduced 1000x when kanamycin resistance was selected; TnpA+TnpB and lacZ-specific ωRNA completely eliminated lac+ colonies. This result is consistent with TnpB being responsible for retention (replacement) of the IS copy following “peel and paste”. Meers et al.,[41] coined the term “peel and paste/cut and copy” for the overall process.
The Copy Choice Model for TnpB Function During Transposition
One of the important questions concerning IS200/IS605 transposition pathway and those of the related IscB-carrying elements had been the way in which these IS maintain their copy number in the donor site following peeling off the donor daughter “chromatid” to leave a transposon-less donor joint in the lagging strand of the replication fork. In the model shown in Fig. IS200.16, excision of a single stranded circular IS intermediate from the lagging strand leaves one double strand copy of the IS in the donor replicon carried by one of the daughter “chromatids” (on the leading strand) and a “donor joint” on the other. In this scenario, no increase in the IS copy number would occur.
The results of Karvelis et al. with ISDra2 provided a solution for this problem which has been extended and supported by a number of authors (see Meer et al.,[41]). Karvelis et al., [72] and Meer et al., [41] proposed a model in which, following excision of the single strand circular IS copy and formation of the donor joint (Fig. IS200.55 A), reRNA/TnpB targeted cleavage of the donor joint is used to initiate a copy/choice replacement of the IS from the remaining IS on the daughter chromatid (Fig. IS200.55 B) for example (see Cox et al., [111]). This would permit maintenance of donor replicon integrity while assuring an increase in IS copy number via transposition. They point out that conceptually, this in some ways resembles group I intron behavior.
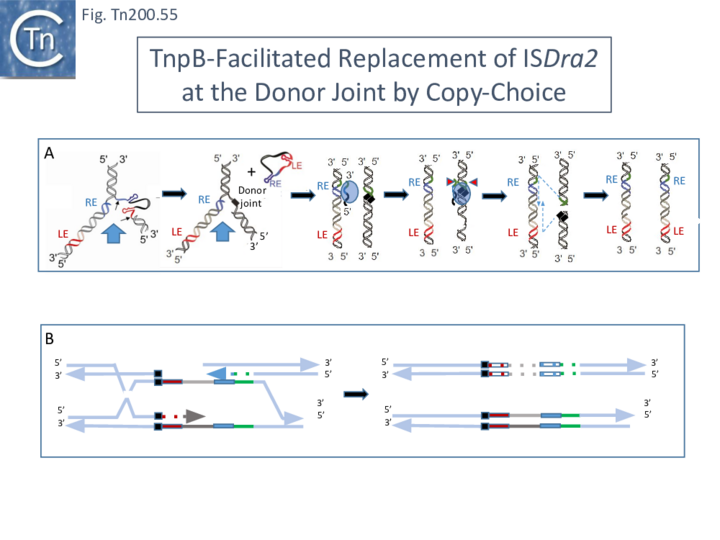
TnpA-mediated transposition of the IS200/IS605 family is well documented. The transposition model is based on in vitro experiments using single-strand oligonucleotides, results from in vivo experiments which implicate DnaG and the observation that the orientation of insertion is correlated with the direction of replication of the target chromosome (see [102]and references therein). The proposed function of TnpB in this process neatly completes the transposition model by offering an explanation of how IS copy number is maintained by replacement of the excised IS at the resulting donor joint.
IStrons
Another role for Y1 transposases was suggested by the identification of chimeric genetic elements widely distributed in the genome of Clostridium difficile[112], the Bacillus cereus group and Fusobacterium nucleatum Subspecies Polymorphum [113][114][115][116] and many other bacterial species [117]: IStrons. These combine functional and structural properties of group I introns at their 5’-end with those of an IS element at their 3’-end (Fig. IS200.56 A and B). This 3' part contains an IS200/IS605 related sequence including two full length or truncated orfs, tnpA and tnpB, very similar to those found in ISDra2 (D. radiodurans) and ISCpe2 (C. perfringens).
IStrons are present at several loci in the same genome, indicating that this element is mobile and may move as a complete genetic unit. All IStron copies analyzed so far are inserted 3’ to the pentanucleotide TTGAT. In vivo, all variants can be efficiently and precisely excised signifying that components necessary for ribozyme activity are present [112]. The data suggest that IS components could mediate the spread of IStron while the intron component could assure splicing.
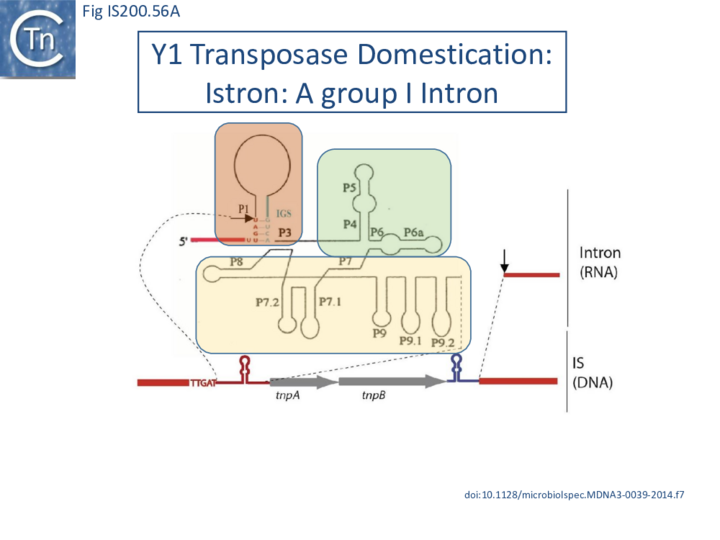
In vitro oligonucleotide-based assays using purified IStron transposase confirmed that at the DNA level, TTGAT is the LE cleavage site in excision and the target site respectively (Caumont-Sarcos, unpublished, cited in He et al.,[102]). At the RNA level, the same sequence is probably required in the splicing reaction[118]. This would represent a novel type of intron invasion and transposition mechanism and provide a direct link between RNA and DNA worlds[102].
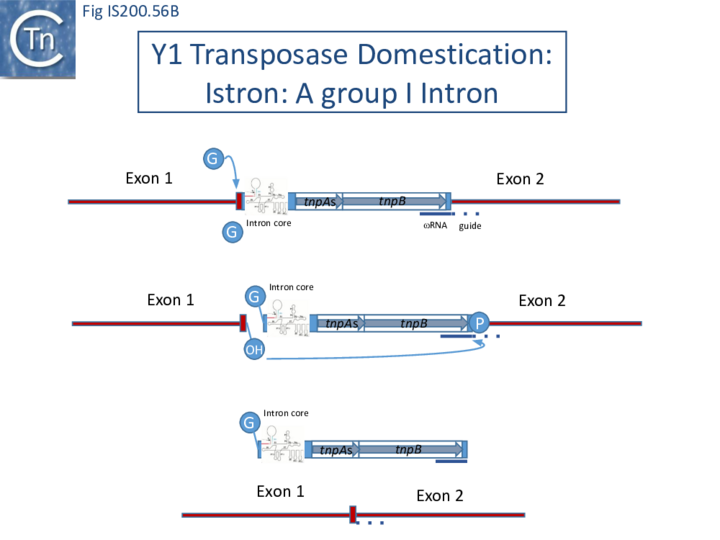
It is interesting to note that related IStrons have now recently been identified which include components of the IS607 family [116][120]. These are characterized by a serine transposase together with a tnpB gene[12].
More recently attention has been focused on the various activities of these IStrons based mainly on the IS607-containing derivatives [117]. These studies have investigated the TnpB activity of IS607 itself and the interplay between DNA transposition, self-splicing intron mobility and RNA guide activity in both IS605- and IS607-based IStrons.
Based on a TnpB phylogenetic tree [41], a bioinformatic analysis for each tnpB gene identified associations with tnpAS (IS607 family), tnpAY (IS200/IS605 family), group I introns (IStron), and ωRNA loci (Fig. IS200.57).
As in the simple IS derivatives, ωRNA loci were invariably located at the left end, 3′ to tnpB in the same region critical for 3’SS (Splice Site) intron recognition. This analysis confirmed the previous observation that not all tnpB-containing IStrons include an intact tnpA.
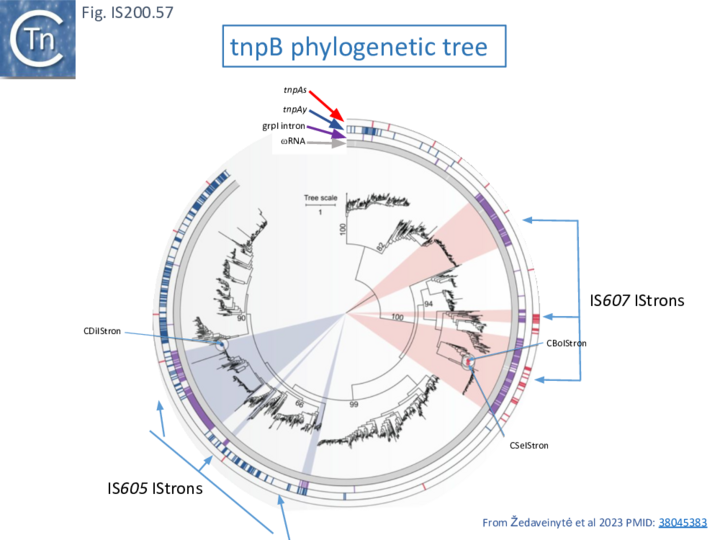
The IS605-based IStron: CdiIStron
The IStron content of Clostridioides difficile (Cdi) and Clostridium botulinum (Cbo), was examined in detail: C. difficile 630 carries 8 IS605 family IStrons (CdiIStron) all without a full length TnpAY (unlike CdISt1 which carries an intact tnpAY gene [121], three freestanding group I introns and three IS605 copies. IStron LE and RE corresponding to those of IS605 were identified using a covariance model developed previously [41] and revealed a TTGAT TAM sequence abutting LE similar to that identified as the CL earlier (see He et al.,[102]) (Fig. IS200.60 A). The overall organization resembled a so-called Twort group I ribozyme [122][123].
Analysis of published RNA sequencing data [124][125] revealed expression from all intron and IS605 elements in the C. difficile genome. Moreover, spliced and unspliced sequences for all but one of the CdiIStron were detected demonstrating that the IStrons are active and defining the exon-intron boundaries. These corresponded perfectly to the predicted IS605 LE/RE. An example is shown in Fig. IS200.62. Here the IStron is inserted into a Toxin Glycosylating Gene, tcdA, in one strain but not in another.
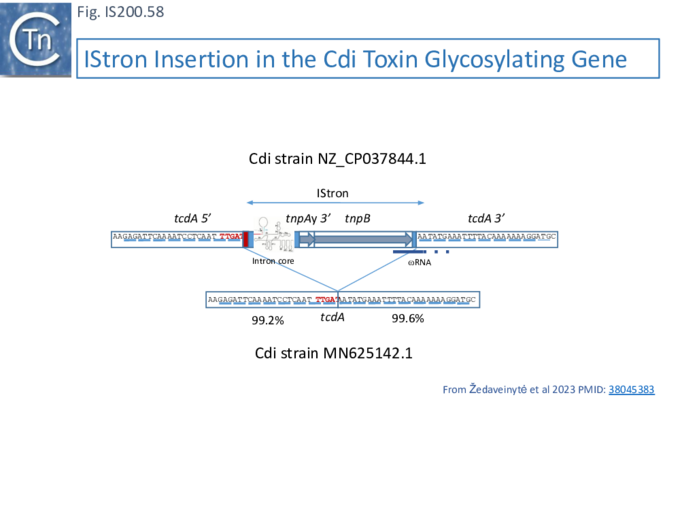
IS607-based IStrons
An IStron including full length tnpAS and tnpB genes (CboIStron; Fig. IS200.59) was identified in C. botulinum strain BKT015925 located on a large botulinum neurotoxin-encoding plasmid together with an IStron lacking tnpA and multiple stand-alone IS607 elements. IS607 elements are difficult to identify since they do not have inverted repeat or palindromic ends and do not generate flanking target repeats (TSD) on insertion. Žedaveinytė et al., [117] were able to detect the LE and RE boundaries using a combination of comparative genomics (comparison of full and empty sites) and homology between CboIStrons. They could define a consensus TAM sequence as TGGG (Fig. IS200.59). Moreover, the covariance model (CM) used to define Cdi group I introns was also be used successfully to detect CBo group I introns and, as in the IS605 IStron, the splice sites overlapped the IS ends. An example of a CBoIStron inserted into a phage antirepressor gene, ant, is shown in Fig. IS200.59.
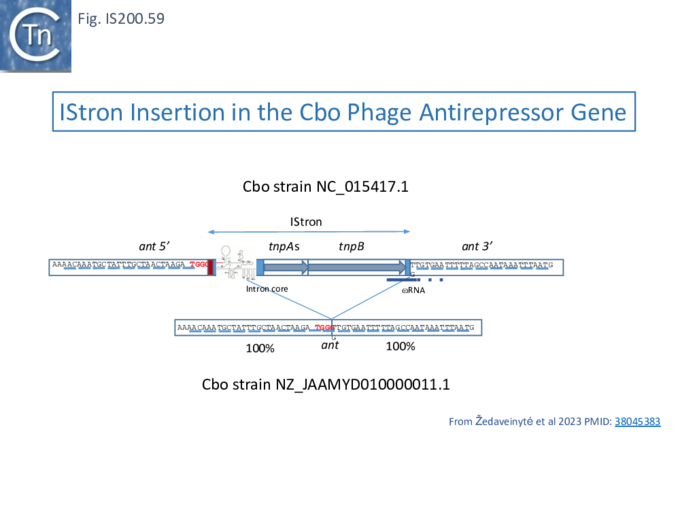
While the transposition reactions of IS605 and IS607 are quite different (TnpAy recognizes secondary structures generated in single-strand DNA in the IS ends while TnpAs recognizes double strand DNA), it had been noted that the ends of IS607 and its relatives included a number of repeat sequences (Fig. IS607.1 and Fig. IS607.2). Identification of IS605 and IS607 ωRNA using the covariance model showed these share common secondary structure features (Fig. IS200.60, Fig. IS200.61 and Fig. IS200.62) [117] comprising three consecutive stem-loop features with a so-called Nexus stem loop (SL1) predicted to facilitate a pseudoknot structure (Fig. IS200.65 and Fig. IS200.66) with the 3’ ωRNA end as has been demonstrated in the case of ISDra2 (Fig. IS200.42; [99]. This appears to be conserved in TnpB systems and in Cas12 guide RNA [78][98][99].
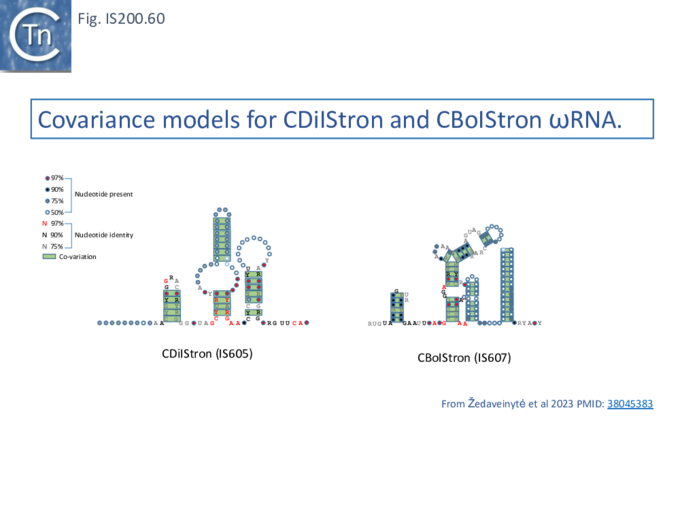
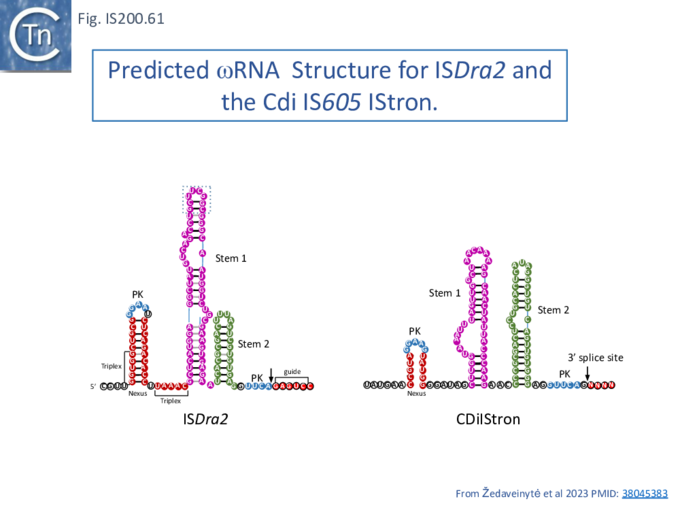
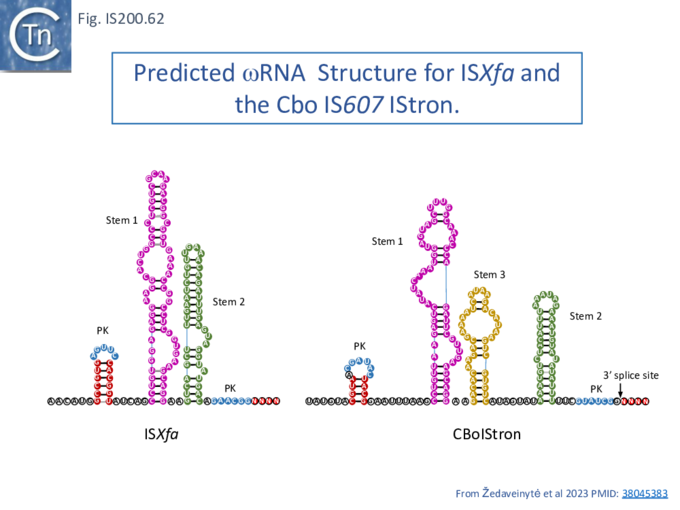
TnpAS IS607 Excision and Insertion Activity
A strong excision reaction, detected by PCR, was observed in E. coli using a “donor” plasmid carrying a mini IS607-based CboIStron lacking both tnpAS and tnpBS and TnpAS expressed from a second plasmid. Circular transposon copies were also observed (Fig. IS607.7). This depended on a functional TnpAS active site and the presence of both IStron(IS) ends. The excision products, which appeared at a frequency of 5% in overnight cultures (several orders of magnitude higher than that catalyzed by TnpAY in an IS605 system under similar conditions; Meers et al., [41] carried a precise donor joint. Internal end deletions showed a requirement for 40 (LE) and 60 (RE) bp for robust excision. In particular, these carry a subset of the repeated elements (3 in LE and 2 in RE) of those identified by Boocock and Rice[34] and Chen et al. [126] (Fig. IS607.2) and implicated in the cooperative assembly of multiple TnpAS dimers into a synaptic complex [126]. Mutation of these repeats individually resulted in reduced excision activity while multiple mutations eliminated excision entirely [117].
Thus, as in the case of IS605, transposase activity of IS607-related transposons, leads to loss from the donor site.
To investigate IS607-IStron insertion, a chloramphenicol resistance tagged suicide (non-replicative) donor plasmid carrying abutted LE and RE and a pir-dependent R6K replication origin, ori, was used: grown in a pir+ strain and transformed into a pir- strain expressing TnpAS (Fig. IS607.8). Cell viability in the presence of chloramphenicol was dependent on TnpAS. Integration specificity was investigated by genome-wide sequencing and found to strictly require a GG dinucleotide with a preference but not an absolute requirement, for the predicted TAM sequence: TGGG.
IStron-encoded TnpB nucleases
Sequencing of RNA in immunoprecipitated CboIStron TnpBS (as has been shown in other TnpB systems; [98]) revealed that it strongly enriched its expected RNA partner. However, there was also a strong signal located 42nt downstream from the covariance model (Fig. IS200.60, Fig. IS200.62). Mutating the RuvC domain resulted in the disappearance of this species suggesting that it is the product of a TnpBS-catalized precursor transcript processing as found for other TnpB systems (e.g. Nety et al. [95]).
Using a plasmid interference assay (see: TnpBGst and IscBGst proteins are active RNA-guided Nucleases; Fig. IS200.54) CboIStron TnpBS showed robust in RNA-guide mediated DNA cleavage and reduced colony formation (i.e. cell viability) by a factor of 105 in a process necessitating target DNA- guide RNA complementarity, a cognate TAM and a catalytically competent TnpB. The reaction was effective using either a natural configuration in which both CboIStron ωRNA and TnpB are expressed from a single transcript or when they are expressed independently.
Defining the CboIStron TAM Sequence: a double role in both nuclease and transposase recognition
An assay similar to that shown in Fig. IS200.40 was used to precisely define the CboIStron TAM sequence required by TnpB during DNA recognition and cleavage. The target plasmid included a randomized 6N library together with the guide sequence and a kanamycin resistance marker. Plasmids carrying the TAM sequence are strongly depleted under selective conditions. The results confirmed the predicted TAM was indeed TGGG (as shown in Fig. IS200.59).
Thus the TnpAS/TnpB system, like TnpAY/TnpB (e.g. Karvelis et al.,[72]) systems have evolved to specify the same DNA motif for nuclease recognition and for transposase recognition.
CboIStron TnpB/wRNA promotes transposon copy number maintenance
Given the similarities of TnpB activities including recognition of the TAM/CLsequence by both transposase and nuclease, it seemed probable that the IS607 CBo TnpB performs the same function.
This was tested using the retention/replacement assay of Meers et al.,[41](Fig. IS200 54 B) where the CboIstron interrupted a plasmid-based lacZ gene and oriented opposite to the direction of transcription of the gene (to avoid the splicing reaction as has been used in assays of transposition of retroelements. Expression of TnpAS alone resulted in transposon loss evidenced by about half lac+ colonies, a frequency reduced about 10x by co-expression of wildtype but not mutant TnpB. The veracity of these results was confirmed by PCR [117].
Busy Ends: Functional interactions between IStron splicing, TnpB and ωRNA
To investigate the interactions between peel and paste/cut and copy transposition and type I intron splicing, a minimal CboIStron lacking both TnpA and TnpB was first examined for its ability to undergo self-splicing in E. coli. The splicing reaction uses exogenous GTP to undergo a transesterification reaction at the 5’SS and a 3’ OH end of the upstream exon which then attacks the 3’SS to form an exon-exon joint and liberate the intron. RT-PCR on extracted RNA revealed both spliced and unspliced products. The spliced product was a perfect joining of the two flanking exons with the exact sequence observed following TnpAS-mediated IStron excision. The reaction required the P7 - P9 catalytic region (Fig. IS200.56 A) and a wildtype 5’SS sequence.
Self-splicing without the intervention of protein factors was confirmed since identical products were obtained with purified RNA.
Coding mRNAs for TnpA and TnpB can be produced from both unspliced and spliced introns, but since the ωRNA scaffold is severed from the guide region, spliced introns are no longer capable of forming functional ωRNAs (Fig. IS200.56 B). Conversely, TnpB-mediated ωRNA processing would separate 5′SS and 3′SS on two distinct molecules, allowing only trans – splicing TnpB - ωRNA binding would also likely obstruct physical interactions required at 3’SS for splicing.
Consecutive deletions in the first 180bp of CboIstron from the ωRNA 5′ end (Fig. IS200.63 Top) dramatically increased splicing. This included most of the ωRNA stem loops which was shown to eliminate TnpB-mediated RNA-guided DNA cleavage. Single or combinations of stem-loop deletions except for stem-loop 5 required for splicing had similar effects. A large change splicing activity (measured as spliced/Unspliced substrate by PCR; Fig. IS200.63 Bottom) for the 180bp deletion implies sequence and/or structural features in this region inhibit splicing in the full length wildtype IStron. The results suggest, perhaps not surprisingly, that the RNA structure alone influences splicing and that splicing and TnpB/ ωRNA activity are negatively correlated.
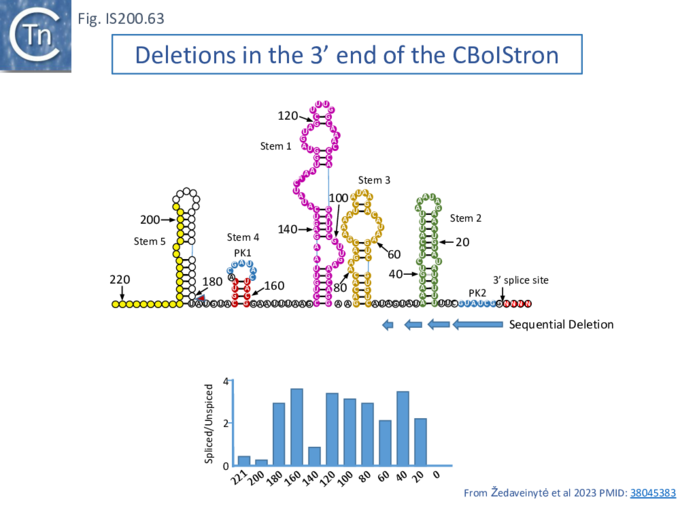
Additionally, the pseudoknot (Fig. IS200.42 and Fig. IS200.63) which appears to be a common feature in ωRNAs and which is essential for TnpB/ωRNA guided DNA cleavage, was observed to play an important role in inhibiting splicing: individual point mutations in PK1 (Fig. IS200.63) which destroy its formation (and eliminate guided DNA cleavage) greatly stimulate splicing activity although mutation in PK2 which is shared by the 3’SS site eliminated both guide activity and splicing.
Moreover, expression of wildtype or catalytically inactive TnpB in trans greatly reduced splicing indicating that TnpB-facilitated ωRNA binding was sufficient for splicing repression. This was further confirmed since TnpB-dependent repression was only observed when most of the ωRNA scaffold was present. It did not occur if ωRNA was replaced by lac RNA.
Busy Ends
There is therefore clearly an intricate balance between the IStron splicing activity and the mutually exclusive functions involved in the cut-and-copy phase of transposition. The end sequences have evolved to accommodate both transposase (either TnpAY or TnpAS), the TnpB/wRNA systems and the self-splicing 5’ and 3’SS. The advantage for the associated IS is that the intron has a wider target choice since it can occur, co-oriented, within coding sequences without consequences for expression for the interrupted gene.
The intricate relationship is apparent from the fact that the ωRNA scaffold is contained within the 3’ intron end but the targeting sequence is contained within the downstream exon. Splicing therefore separates the guide and scaffold sequences. The balance between splicing and guided target DNA cleavage is modulated both by TnpB- ωRNA binding which, by occlusion, prevents the access of the upstream exon to the 3’ phosphate bond (Fig. IS200.56B) and by the ωRNA itself by its pseudoknot formation which competes for the 3’ splice site. An overview is provided in Fig. IS200.64 taken directly from Žedaveinytė et al.,[117].
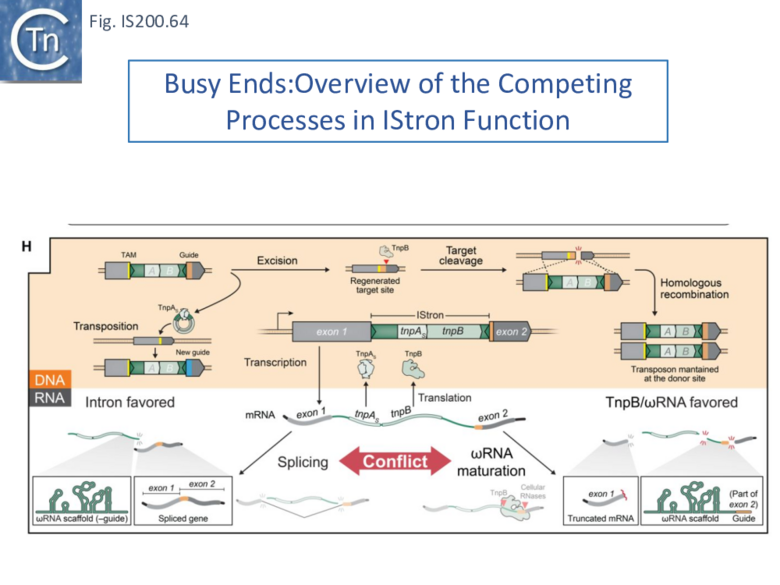
The Eukaryotic Connection: Fanzor eukaryotic TnpB relatives
Fanzor proteins are eukaryotic relatives of TnpB first identified in a bioinformatics search [80]. The first, SPu-1-1p (633-aa), was identified in a fungus Spizellomyces punctatus. The single Orf was flanked by 33-bp Terminal Ivertead Repeats (TIRs) and a putative 2 bp TSD (TA). The 2,100-bp long SPu 1 element was found in 17 full length copies and homologues were also identified in a number of other eukaryotes including metazoans, fungi, protists and dsDNA viruses infecting eukaryotes. They are very distantly related to TnpB from both the IS200/IS605 and IS607 families with which they share 15% identity over the 300 aa C-terminus (Fig. IS200.55). This comprises a number of highly conserved residues including the TnpB Zn finger (Fig. IS200.30, Fig. IS200.31, Fig. IS200.36, Fig. IS200.47).
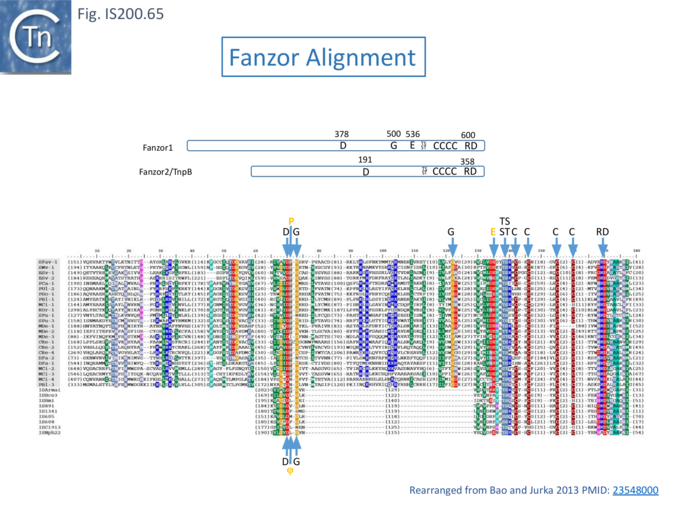
A phylogenetic tree (Fig. IS200.56) showed that Fanzor1 formed a well separated clade and that Fanzor2 was associated with some, but not all, TnpB proteins.
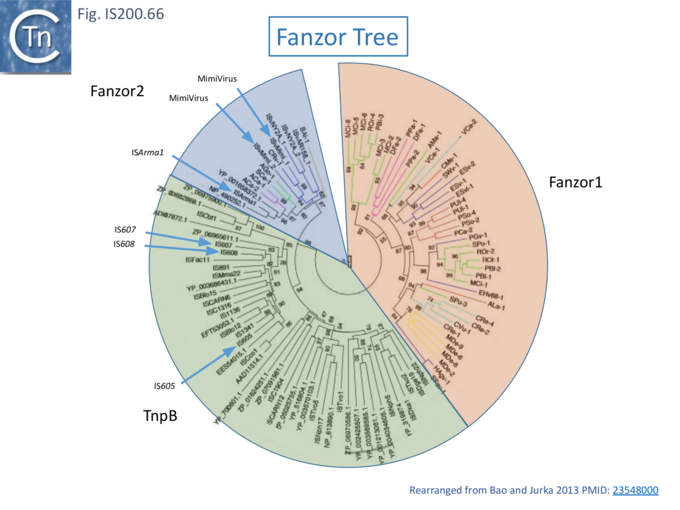
TnpB Clade
This is restricted to prokaryotic elements although a later analysis [79] identified TnpB which are closely related to Fanzor2 (pro-Fanzor) and mainly found in cyanobacteria.
Fanzor1
Fanzor1 which is more distantly related to TnpB (Fig. IS200.66) was observed to be associated with a number of different TE including so-called IS4 -type elements in the alga Ectocarpus siliculosus and its virus, virus 1 and Sola2 elements from the slime moulds Dictyostelium fasciculatum and Polysphondylium pallidum, Tc/mariner, Helitrons, MuDr relatives and several insect viruses. All these examples are from eukaryotes.
Fanzor2 and/or Fanzor1 are of bacterial origin
On the other hand, Fanzor2 proteins are found associated with serine recombinases as in IS607. It should be pointed out that most of these are from Giant viruses or nucleocytoplasmic large DNA viruses (NCLDVs) that infect algae (Phycodnaviruses) and amoebae (Mimivirus). It has been demonstrated that such viruses acquire genetic information from ingested/infecting bacteria [127][128][129]. Indeed, several of these had been identified earlier as IS607 derivatives which maybe of bacterial origin. These appear to be a different subclade to the majority of bacterial examples although a few prokaryotic elements are associated (Anabaena sp. PCC 7120; ISArma1; and Microcystis aeruginosa NIES-843).
A more extensive analysis based on a much larger sequence library [130] identified more than 3000 representatives of the TnpB superfamily. These were chosen based on structural mining of an AlphaFold database and sequence profiling of the non-redundant NCBI database and grouped these into a phylogenetic tree (Fig. IS200. 67). The overall topology is similar to that described by Bao and Jurka [80] the eukaryotic examples fall in two major groups comprising Fanzor1 and Fanzor2.
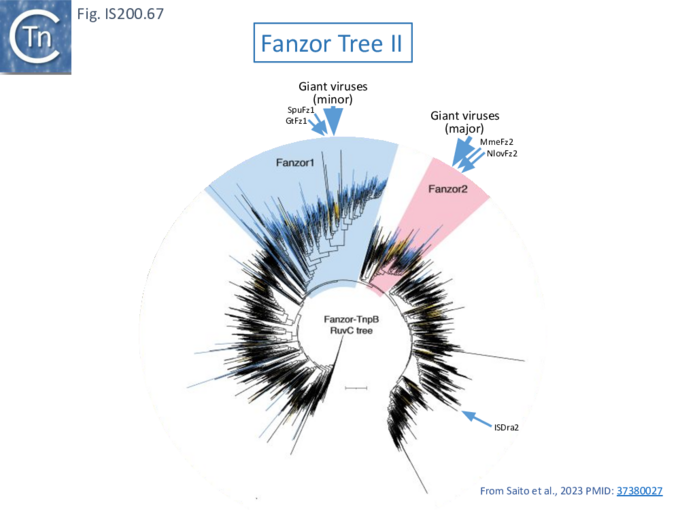
Fanzor1 is found in fungi, but also in protists, eukaryotic viruses, in particular giant viruses where there is a close association with internalized bacteria since these infect hosts living in symbiosis with bacteria (see Filee et al., [127]), arthropods and plants. Fanzor2 is also found in several giant viruses and in choanoflagellates which also feed on bacteria (and viruses) as well as in Stramenopiles, Alveolates and Rhizaria which may also ingest bacteria.
The authors manually examined eukaryotic branches (radiations), and sometimes simply single leaves, emerging from other TnpB branches around the tree [130]. These showed that they were also from hosts featuring lifestyles intimately connected to bacterial species (e.g. bacterivores or living with parasitic bacteria).
These data were interpreted to suggested that the Fanzor proteins, FZ1 and FZ2, were originally acquired from bacterial hosts possibly twice [130][131]. It should be noted that those Fanzor2 proteins which have similar spacing to TnpB (Fig. IS200.65) and were attributed a eukaryotic association, are now thought to be misclassified prokaryotic proteins [79] moreover, Yoon et al.,[79] could find no support for independent evolution of Fanzor1 from prokaryotic Fanzor1-like derivatives.
They show a similar domain arrangement with increasing complexity from the closely related TnpB such as that of ISDra2 and the Fanzor2 proteins (Fig. IS200.68), through an expansion of the HTH (Rec) region in the Fanzor1 derivatives to the extensive expansion found in the Cas12a proteins (Fig. IS200.68).
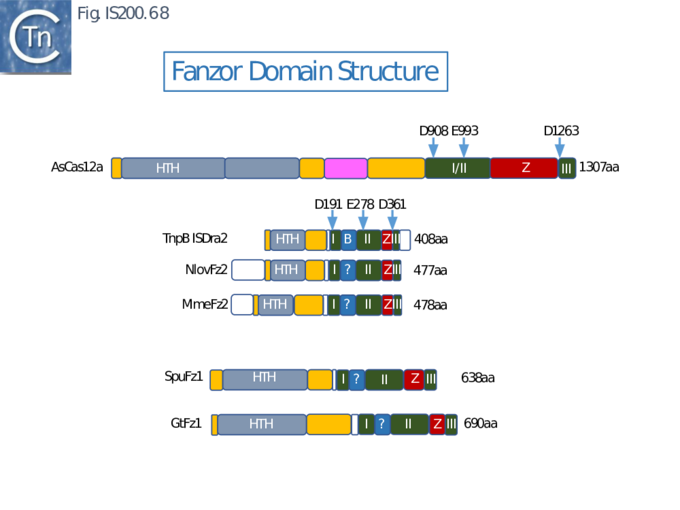
Fanzor2 and/or Fanzor1 may have evolved from an IS607 ancestor
It has been proposed that Fanzor evolved from a clade of IS607-related elements [79] with an unusual active site configuration. Interestingly, IS identified in the Mimi NCLDV, ISvMimi_1 and ISvMimi_2 in ISfinder (NC_006450), had already been identified as related to IS607 based on their transposase, tnpA, genes but carry a tnpB-like downstream gene which is much longer than typical tnpB [127][132].
Two features distinguish TnpB and Fanzors in spite of their sharing similar domain organizations [79]; summarized in Fig. IS200.69 A): firstly, the Fanzor RuvC1 catalytic D is followed almost exclusively by a proline (GPG; Fig. IS200.55) whereas in TnpB there is typically a hydrophobic residue, φ (where φIcan be: , L, F, W, Y and M), instead (DφG; Fig. IS200.55; Fig. IS200.35; Fig. IS200.36); secondly, TnpB RuvC2 typically contains a catalytic glutamate situated ∼ 50 residues up- stream of the ZF motif which they call Ecan for cannonical (Fig. IS200.35; Fig. IS200.36; Fig. IS200.58) whereas in the Fanzor RuvC2 this glutamate is six residues upstream of the ZF motif, which they call Ealt for alternative (Fig. IS200.55; Fig. IS200.59). Yoon et al., [79] also suggest that Fanzors with the E spacing typical of TnpB and previously interpreted as novel Fanzor subtypes [80][130] appear to be prokaryotic TnpBs that had been mis-annotated as eukaryotic Fanzor2.
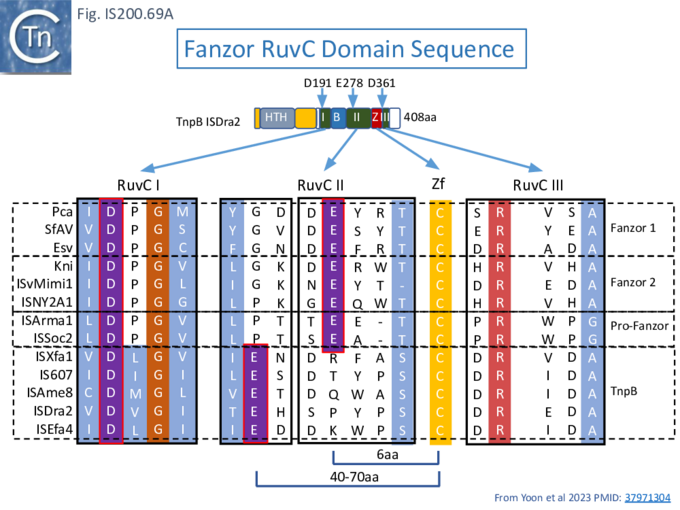
Fanzor1 may have evolved from Fanzor2
However, when about 800 TnpB homologues obtained by database mining were used to create a maximum likelihood tree based on their RuvC features, Yoon et al., [79] observed a clear separation between RuvC II Ecan (TnpB) and Ealt (Fanzor) containing sequences (Fig. IS200.69 B) with those carrying Ealt further distributed into two clades with RuvC I DG (TnpB) or DPG (Fanzor). A small number of TnpB-like proteins were observed to be closely related to Fanzor2, mainly from cyanobacteria, are associated with IS607 TnpA also closely related to those associated with Fanzor2, and were called ‘pro-Fanzors’ (Fig. IS200.69 B).
Yoon et al.,[79] could not find compelling evidence that Fanzor1 was acquired directly from a prokaryote and it seemed possible that it might have evolved from an acquired Fanzor2 protein. Bao and Jurka [80] observed that Fanzor1 can be found in a number of eukaryotic transposons such as Tc/mariner, Helitrons, and, associated with what the authors call IS4-type Tpases in ESvi1B and ESv2 (brown algae Ectocarpus siliculosus (see Filee et al., [127] [132] for an early description of NCLDV-associated IS). However, it was thought that Fanzor2 was limited to prokaryotic IS607-like elements [80]. To examine this further, IS607 derivatives encoding Fanzor1 and eukaryotic transposons carrying Fanzor2 were searched in the updated library [79].
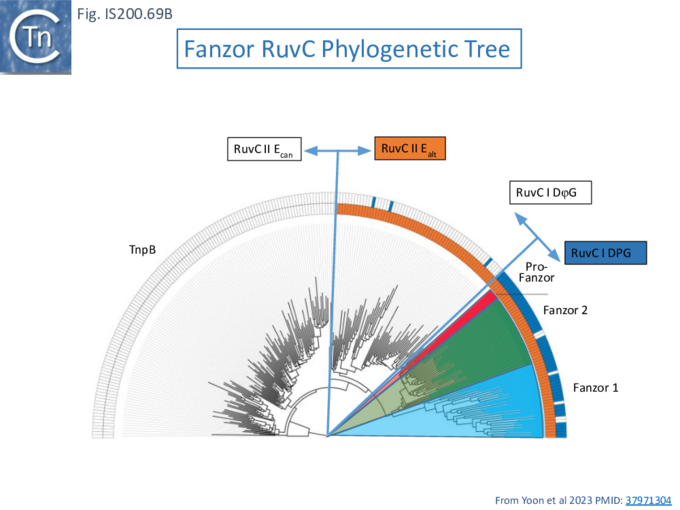
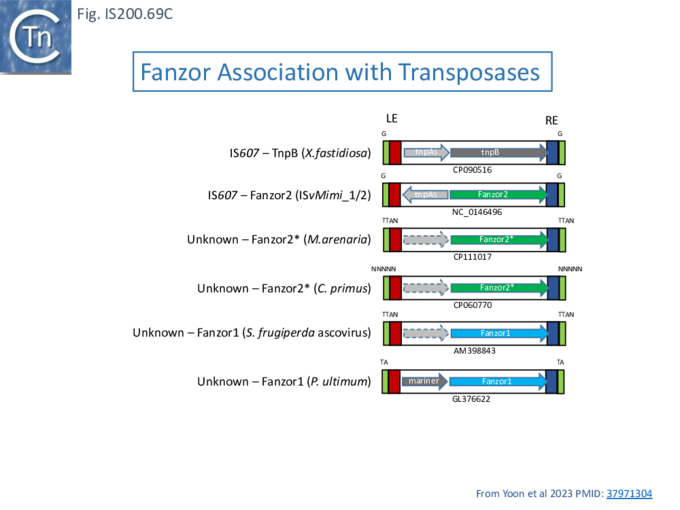
As expected, the results identified a number of Fanzor1 associated with a number of eukaryotic transposons but failed to identify IS607-associated Fanzor1. On the other hand, a number of Fanzor2 were found to be associated with non-IS607 eukaryotic elements of different families (Fig. IS200.69 C) from organisms such as the algae Chloropicon primus and various mollusc species including Mercenaria mercenaria. These were called Fanzor2* to distinguish them from the “prokaryotic” Fanzor2.
The authors proposed that an ancestral Fanzor2 gave rise to Fanzor1 based on: the conserved RuvC profiles; the absence of prokaryotic Fanzor1 proteins; and that distantly related Fanzor2 was found in different eukaryotic transposon suggesting that it had been captured several times. Moreover, the absence of a detectable close evolutionary link between Fanzors and IS200/IS605 TnpBs is noteworthy since since IS200/IS605 family members appear to be more abundant than IS607 derivatives [41]. This suggested that specific features of IS607 TnpB might have facilitated their evolution in eukaryotes [79] (see Is IS607 TnpB the Ancestor of Fanzor Proteins?).
Although the Fanzor proteins are very widely distributed in the eukaryotic world (Fig. IS200.67) and are sometimes found associated with potential transposable elements and in multicopy, their function has yet to be clearly established.
Fanzor Activity
Saito et al.,[130] used two FZ1 examples (from the soil fungus S. punctatus , SpuFz1, and the alga G. theta, GtFz1) and 2 FZ2 (from N. lovaniensis, NlovFZ2, and the marine mollusk M. mercenaria, MmeFZ2) for functional studies.
Comparison of Alphafold structural predictions of FZ proteins with known structures of ISDra2 (PDB: 8H1J) and AsCas12a (PDB: 5B43) showed that despite a large sequence and length variation (Fig. IS200.58) all six proteins share a common “core” domain including a WED and RuvC region.
A predicted active catalytic site formed by positively charged residues is found in the RuvC region in ISDra2 TnpB, SpuFz1, GtFz1, NlovFz2 and MmeFz2. However, the core regions include various family-specific insertions. The Cas12 protein, AsCas12a (1307aa), carries a 900aa insertion in the WED domain, the REC region, which forms a protective channel for the spacer–target RNA-DNA heteroduplex region and is likely to be involved in this R-loop formation. It is reduced to three helices (100aa) in TnpBDra2, NlovFz2 and MmeFz2 (see Fig. IS200.34) and probably serves the same function. NlovFz2 and MmeFz2 are very similar to I TnpBDra2, but each harbors a unique amino-terminal disordered region, with NlovFz2 featuring a 96aa and MmeFz 61aa segment.
Characterization of the guide RNA system followed relatively established procedures: identification of the associated ωRNAs from the RE region of the FZ orthologues expressed in S. cerevisiae by small RNA-seq for RNPs and secondary RNA structure prediction.
Initially, SpuFz1 [80], a single open reading frame (ORF) flanked by well-conserved 30bp terminal repeats was used for further functional studies. S. punctatus DAOM carries 42 copies of this 2.1-kilobase pair Spu1 transposon: 19 with full length or remnants and 134 lacking the FZ orf which they call “ghosts” but which are equivalent to previously described MITES. RNA-seq revealed an 88–90-nt ncRNA species downstream of Fz in several S. punctatus loci which could also be identified in pulldown experiments in Saccharomyces cerevisiae. These included 14–15 nt of variable sequences beyond the conserved 75-nt region at the 3′ end. This was repeated with GtFz1 and with an Fz1 locus from G. theta, four Fz2 loci from N. lovaniensis, and two Fz2 loci from M. mercenaria.
Purified complexes of all four proteins (SpuFz1, GtFz1, NlovFz2 and MmeFz2) with their ωRNA were used in an assay (e.g. Fig. IS200.40) to define the associated TAM sequences (SpuFz1, CATA; GtFz1, TTAAN; NlovFz2, CCG; and MmeFz2, TAG) and to identify cleavage points on the non-target and target strands (NTS and TS) in a target DNA. These varied according to the protein generating: 5’ overhangs (SpuFz1), 5’ or 3’ overhangs or blunt ends (GtFz1), blunt ends (NlocFz2) or 3’ overhangs (MmeFz2). Finally, the structure of the SpuFz1 RNP complex with its target DNA was obtained by cryo-em and, as expected, was found to consist of an SpuFz1 monomer associated with a single ωRNA molecule together with the target DNA.
Functional Relationship Between Fanzor Evolution and IS607 TnpB
Yoon et al., [79] suggested that specific features of IS607 TnpB might have facilitated their evolution in eukaryotes. The most obvious major difference that IS200/IS605 family members use a single strand circular DNA intermediate whereas IS607 uses a double strand circular intermediate.
However, they also noted that while IS200/IS605 family members, insert downstream of a short motif (Fig. IS200.3, Fig. IS200.5, Fig. IS200.7) [22][102], IS607 family members typically insert via recombination between matching dinucleotide motifs (Fig. IS607.6) [34][126]. This means that for IS200/IS605 members, the first nucleotide after the right end (or the ‘right-flanking nucleotide’) is variable whereas in IS607 members it is fixed (G in Fig. IS607.6).
The nucleotide abuting the IS200/IS605 right end corresponds to the start of the guide sequence in TnpBs [72] and it was reasonable to determine whether this is also true for IS607. TAM depletion assays (which uses a target plasmid carrying a library of potential TAM sequences abutting a guide sequence e.g. Fig. IS200.44 ii) in which TnpB Recognition of a TAM/target site results in depletion in the TAM carrying bacterial sub-population was used to investigate this. Using re(ω)RNA variable boundary mutants of ISXfa1, an IS607 member from Xylella fastidiosa, (see also Žedaveinytė et al., [117]) it was observed that the RE abuting G nucleotide behaved as part of the re(ω)RNA scaffold rather than as part of the guide as has also been observed by Žedaveinytė et al for an IStron IS607 derivative [79][117] .
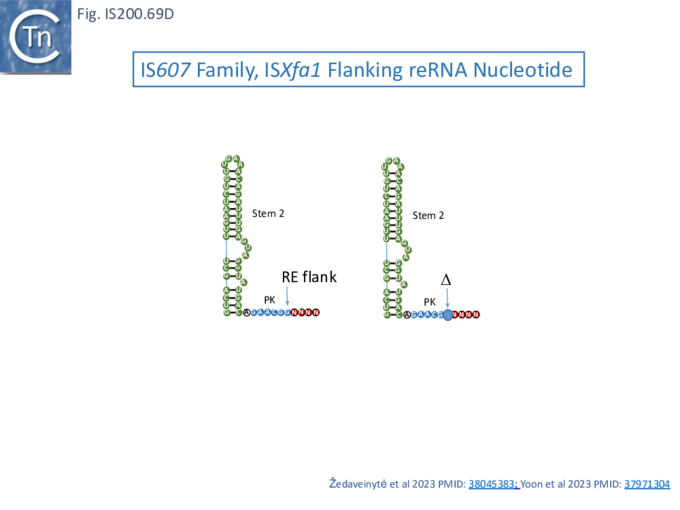
Analysis of ISXfa1 reRNA by Žedaveinytė et al., [117] (Fig. IS200. 53) and by Yoon et al., [79] gave similar results with potential stem-loops and, like other re(ω)RNAs, including a pdeudoknot essential for activity. Based on similarities and differences between the reRNAs and from structural models, it was proposed (Fig. IS200.59 E) that TnpB from an ancestor of IS607 gave rise to Fanzor1 (for example, that found in ISvMimi1) via an intermediate (Pro-fanzor) and that Fanzor2 derived from a eukaryotic Fanzor1.
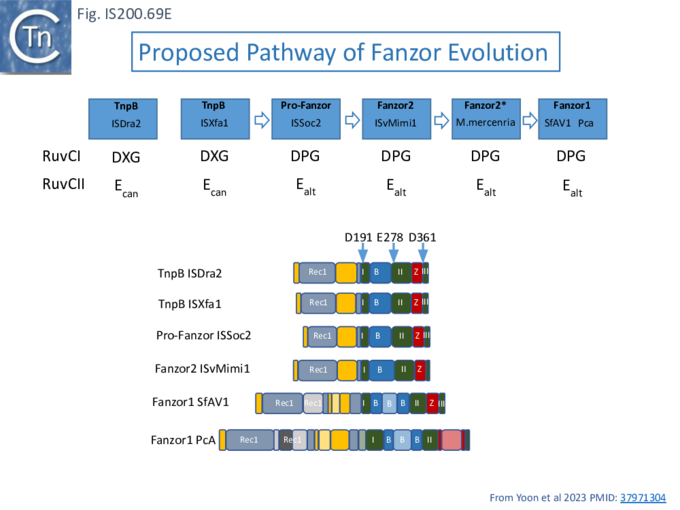
Y1 transposase domestication
There are many examples of eukaryotic transposases whose activities have been appropriated to perform various cellular functions (see [133][134][135]. However, the very few examples of this domestication for prokaryotic enzymes concern Y1 transposases.
TnpAREP and REP/BIME
Recently, a new clade of Y1 transposases (TnpAREP) was found associated with REP/BIME sequences in structures called REPtrons [136][137] (Fig. IS200.70 A). In spite of their compact size, bacterial genomes carry many repetitive sequences, often important for genome function and evolution. Among them, Repetitive Extragenic Palindromic sequences (or REPs) are short DNA repeats of 20-40 bp that can form stem-loop structures preceded by a conserved tetranucleotide (GTAG or GGAG) (Fig. IS200.71). REPs are found in intergenic regions in many bacterial species, particularly in proteobacteria, at high copy number [136][138][139].
There are nearly 590 copies in Escherichia coli K12[140] (Fig. IS200.42) and up to 2200 copies in Pseudomonas sp GM79[139]. REPs can exist as individual units but can cluster in more complex structures called Bacterial Interspersed Mosaic Elements (BIME). These are composed of two individual REPs in inverse orientation (REP and iREP) separated by a short linker of variable length. BIME are often found in consecutive tandem copies (Fig. IS200.70). Several roles have been attributed to these sequences including genome structuring, post-transcriptional regulation and genome plasticity. REPs are known to interact with protein partners such as Integration Host Factor[141], DNA gyrase[142] and DNA polymerase I[143].
REPs also increase mRNA stability and can act as transcriptional terminators [138] or as targets for different IS [15][144]. It has also been suggested that REP sequences are involved in REP sequences can downregulate translation of upstream genes dependent on trans-translation. This occurs only if they are within 15 nt of a termination codon. It has been suggested that that REPs can stall ribosomes, leading to mRNA cleavage and induction of the trans-translation process[145]. Recombination at REP sequences has also been shown to be involved in the formation of F’ plasmid derivatives (the classic F plasmid carrying various portions of the chromosome (Fig. IS200.72) from Hfr strains[146]. However, the origin of REPs and their dissemination mechanisms are poorly understood.
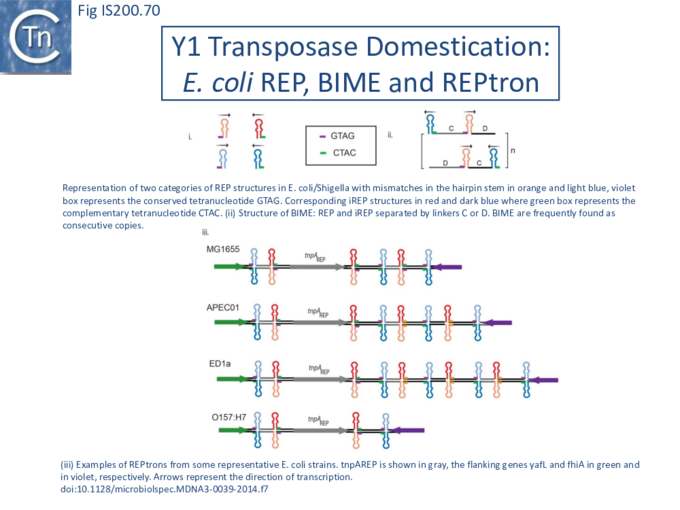
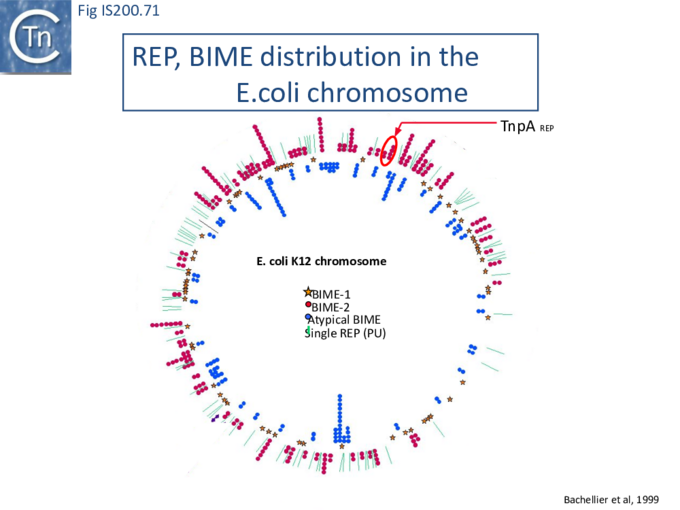
Although more complex, REPtrons are reminiscent of IS200 group members (Fig. IS200.70). However REPtrons do not appear to be mobile and, in general, a single copy of a given REPtron co-exists with numerous corresponding REP/BIME and genomes may harbor several distinct REPtrons[136][139]. It has therefore been suggested that REP/BIMEs represent a special type of non-autonomous transposable element mobilizable by TnpAREP.
In vitro analysis of REPtrons: Analysis of E. coli REPtron activity in vitro has shown that, like TnpAIS200/IS605, TnpAREP strictly requires single stranded REP/BIME DNA substrates and is strand specific, only REP can be processed, whereas iREP are refractory to cleavage [137]. Purified E. coli TnpAREP promotes ssREP cleavage (in the linker sequences either 3’ or 5’ to the REP structure) and rejoining, and this activity requires the conserved tetranucleotide GTAG and the bulge in the middle of the REP stem [137][148]. Cleavage in vitro is less specific than that of TnpAIS200/IS605 and occurs at a CT dinucleotide.
In contrast to TnpAIS608 and TnpAISDra2, E. coli TnpAREP is a monomer in solution and in the crystal structure[148]. Moreover, in the co-crystal structure, the short C-terminal tail is inserted into the active site blocking access to an ssDNA. It may, therefore, play a regulatory role in the activity. Indeed C-terminal truncation of TnpAREP resulted in increased cleavage activity relative to the full-length protein in vitro. The biochemical and structural analysis suggested that the GTAG 5’ to the foot of the REP hairpin may play a similar role to the guide sequences GL/R in IS200/IS605.
Moreover, structural data also highlighted numerous specific contacts between TnpAREP and GTAG, explaining its importance in the activity and clearly distinguishing TnpAREP from TnpAIS200/IS605, which do not directly contact the guide sequences (Cleavage site recognition). The way by which TnpAREP promotes REP/BIME proliferation through their host genomes remains to be determined.
IS200 Regulation and Salmonella Pathogenicity
Although IS200 is present in moderate to high copy number in certain bacteria (e.g. Salmonella typhimurium 5-12 copies and Salmonella typhi 26 copies; and the IS200 family member, IS1541, in Yersinia pestis >50 copies), it appears to be recalcitrant to transposition [149] (The IS200 group) and exists in a “dormant” [150] state. Although samples taken 30 years apart showed no changes in IS200 patterns [149][151][152], there is evidence that the closely related IS1541 element is active in facilitating mouse infection by Y. pestis [150]. The high copy number, low transposition frequency and little accumulation of mutations would imply the existence of a robust selective pressure to maintain the IS [153].
Transposase expression is regulated by an antisense RNA
IS200 elements express two small RNA molecules, a tnpA-encoding mRNA, mRNAtnp (Fig. IS200.73) and a small antisense RNA, asRNA. Regulation of transposase expression occurs at several levels: IS200 LE includes a pair of inverted repeats which constitute a strong, bidirectional, r-dependent terminator (Fig. IS200.73a and b) reducing impinging transcription from entering the IS by ~85%; in addition, LE-proximal mRNA secondary structure sequesters the Shine-Dalgarno sequence (SD) (Fig. IS200.73 b and d) which inhibits tnpA translation by a factor of 20; and a small antisense RNA, asRNA (also called art200; [154] which, by pairing with mRNAtnp, reduces translation 15 fold. A promoter for asRNA, PA, was identified which, when mutated, reduced art200 expression in an E. coli host. Additionally, direct binding of the chaperone RNA binding protein Hfq to a region upstream of the ribosome binding site also occludes ribosome binding [154].
TnpA expression has been investigated using a lacZ translational fusion (codon 10) with tnpA (codon 60; Fig. IS200.73 c). Reducing art200 expression using a promoter mutant (PA-6) led to a significant increase in lacZ expression (~13 fold compared to the wildtype IS200 sequence). Also, when a lacZ fusion with a wildtype IS200 PA sequence was challenged with constructions carrying a 5’ tnpA mRNA segment (nts 45-298; tnpAtrunc-wt; Fig. IS200.73 c) complementary to asRNA and under control of a moderate (Ptet) or a strong (PT7) promoter, a significant increase in lac expression occurred. This presumably resulted from titration and degradation of art200 by over production of its tnpA RNA complement. Use of a tnpAtrunc-M1 mutant, unable to pair with art200, failed to show this response indicating that RNA-RNA pairing is necessary. Moreover, supplying art200 in trans, produced from its own promoter to the lacZ translational fusion with a IS200 PA-6 mutation also reduced lacZ expression.

Transposase expression is regulated by an antisense RNA and Hfq
The involvement of Hfq in art200 RNA/mRNA interaction was suspected from results obtained with asRNA (RNAout) of IS10 from the transposon Tn10 in which Hfq was found to promote antisense pairing with the transposase RNA (Tn10; [155]. art200 RNA was identified from Hfq immunoprecipitation (Hfq-IP) data sets as an asRNA (called STnc490 by the authors) complementary to 90nt of the 5’UTR (untranslated region upstream of the tnpA gene; Fig. IS200.73) of IS200 in Salmonella [156] [157]. A similar RNA from the closely related Yersinia pestis IS1541 has also been identified [158].
The involvement of Hfq in transposase (lacZ) expression (Fig. IS200.73 ci) was confirmed by performing the E. coli titration (Fig. IS200.73 cii) and trans complementation experiments with art200 produced from its native promoter (Fig. IS200.73 ciii). Experiments carried out in isogenic hfq+ and hfq- strains showed that: in the context of a wildtype PA sequence, LacZ expression was 5 fold higher in the absence of Hfq indicating that Hfq represses TnpA expression; the hfq- and PA-6 mutations act synergistically in derepressing TnpA expression; and art200 supplied in trans could repress the expression in the PA-6 mutant independently of the Hfq status of the host [154].
Additionally, it was demonstrated that RNA pairing occurs in vitro. Lead (Pb2+) acetate “footprinting” (Fig. IS200.73 d) was carried out on a mixture of the two prefolded RNA molecules. The art200 secondary structure and interaction with the 5’- fragment of transposase RNA was probed by RNA footprinting in the presence and absence of a tnpA RNA fragment (tnpA1-173; see Fig. IS200.73 d). Specific residues of each RNA became refractory to cleavage indicating a transition from single-stranded to a double-stranded state: a number of art200 residues showed reduced Pb2+ sensitivity in the presence of tnpA1–173 and in tnpA1-173, certain residues showed strong decreases in reactivity to RNase A (which degrades single-stranded RNA at C and U residues) or T1 (specific for at G residues in single-stranded RNA), or strong increases in V1 (which cleaves base-paired nucleotides) on art200 addition. This occurred on the upper part of the art200 RNA stem loop Fig. IS200.73 d left green) on addition of the tnpA1-173 RNA and on the upper part of the tnpA1-173 stem loop Fig. IS200.73 Ad right red) on addition of art200 in a reciprocal experiment [154].
These observations are consistent with a model, shown in Fig. IS200.73 e, in which the two RNA molecules initiate the interaction at the tips of the stem loops [154] followed by propagation of base pairing to the left and right sides facilitated by Hfq. This base-pairing occludes the 30S ribosome binding site (as was demonstrated in vitro) and inhibits TnpA expression in vivo [150][154] .
It should be noted that even in the absence of asRNA, Hfq binds upstream of the ribosome binding site and prevents 30S binding directly to tnpA1-173 RNA.
What is the impact of IS200 on its host genome?
As a quiescent insertion sequence which carries no passenger genes, it was argued that IS200 probably does “not contribute transposition-dependent functions to the host” [150] but its extreme stability over long time periods suggests that it might contribute important functions to its host. In a subsequent study, the Haniford lab raised the possibility that this function may be related to the RNA it expresses since small RNAs are known to play an important role in the control of bacterial cell processes often facilitated by Hfq (e.g. [159][160][161][162][163][164][165][166][167][168].
Although there are multiple layers of regulation which lead to low levels of tnpA translation, tnpA expression is relatively significant. In addition to the maintenance of some IS200-driven transcription, art200 expression appears to be growth phase regulated, increasing during S. typhimurium transition into stationary phase in rich medium and in growth media which stimulate Salmonella Pathogenicity Island (SPI) expression [156][154]. Additionally, art200 expression increases in stationary-phase while tnpA RNA expression decreases ∼ 5-fold.
Which host genes might be regulated by IS200 RNA?
One interesting possibility is that these RNAs are in some way involved in regulating various processes in the host cell [150] and evidence was obtained from RNA-seq experiments that the tnpA 5’UTR RNA acts as a repressor of a number of host genes by base pairing.
In these experiments, the levels of IS200 RNA in S. typhimurium was modified in various ways. By introducing a plasmid carrying a truncated tnpA mRNA derivative, tnpAtrunc WT-255 (including nt 1-255), highly expressed constitutively from a Ptet promoter, the levels of art200 RNA could be reduced by titration and degradation of the base-paired tnpA mRNA and art200 RNA. RNA-seq revealed 187 genes whose transcription was altered under these conditions. To determine whether the effect was due to depletion of art200 or to high tnpAtrunc WT-255 levels, a mutant, tnpAtrunc M1-255, which prevents initiation of pairing (M inFig. IS200.73 d and e), was used: art200 RNA-affected genes were expected to show differential expression with highly expressed tnpAtrunc WT-255 but not with tnpAtrunc M1-255 while tnpAtrunc-regulated genes would show differential expression with both tnpAtrunc WT-255 and tnpAtrunc M1-255 compared to the empty vector plasmid.
Overexpression of tnpA had a far greater effect on gene expression than did depleting art200: 77 genes were differentially expressed in the presence of either tnpAtrunc WT-255 and tnpAtrunc M1-255 while 6 were repressed by art200 (glnH , gltI , acs , icdA , hutU and fadR)[150]. Among the tnpAtrunc RNA-regulated genes, a number were located on the Salmonella Pathogenicity Island (SPI-1) and are involved in virulence: in particular the sipABC effector (translocon) proteins involved in cell invasion during Salmonella infection (e.g. [169]), was repressed. The fact that these were affected by both tnpAtrunc WT-255 and tnpAtrunc M1-255 indicated that their expression is directly influenced by tnpA itself and does not depend on pairing with art200.
5’UTR RNA is processed.
Since the model bacterium S. typhimurium strain LT2 is not virulent, Ellis and coworkers [150] subsequently examined a virulent strain, SL1344, in some further studies. This carries 7 IS200 copies instead of the 6 carried by LT2.
It was thought more likely that, instead of the entire 5’UTR of tnpA mRNA, the true regulator might be a processed form of this RNA. Total RNA was examined by Northern blot from the wildtype SL1344 strain and from a derivative in which one of the chromosomal tnpA genes had been fused to a tet promoter, Ptet , providing constitutive expression. Three RNA species of ∼ 90 nt, ∼ 110 nt and > 310 nt were observed using a 5’UTR probe (Fig. IS200.74). They could not be detected when 4 of the 7 IS200 tnpA copies were removed but overexpression of tnpAtrunc WT-255 resulted in the reappearance of both the 90 nt and 110 nt species indicating that both are located within the first 255 nucleotides of the tnpA mRNA [150].
Primer extension studies combined with TEX treatment (Terminator 5’ monophosphate dependent Exonuclease) which would remove processed RNA, revealed two processing sites (A and B in Fig. IS200.74a) at nts 19 and 108 Fig. IS200.74b).
The processed RNA represses SPI-1 genes by repressing invF transcriptional activator transcription.
Cloned derivatives carrying the first 5’ NTR 50, 200 and 250 nts (tnpA50, tnpA200 and tnpA250) all repressed the translocon genes sicA, sicB and sicC by a factor of ~2.5 but not expression of a control gene, thrS. Interestingly, tnpA50 gave the strongest effect. It is the only derivative unable to pair with art200. All three tnpA RNAs but, in particular tnpA50, were also found to reduce expression of invF mRNA as did high tnpAtrunc expression. InvF is an SPI-1-encoded transcription factor which activates the large SPI-1 T3SS (Type III Secretion System) translocon operon which in turn promotes entry into the intestinal epithelium in the course of an infection.
The effect of this on S. typhimurium SL1344 invasion of HeLa cells showed that tnpA overexpression resulted in a reduction in invasion by a factor of 2 compared to the wildtype strain[150].

Direct tnpA RNA-invF RNA Interaction in vivo and in vitro.
Although no complementarity was found between tnpA RNA and any sequences within the large T3SS operon, an extensive complementarity was identified between 5’ tnpA transcript nts 1- 63 and nts 104-160 upstream of the invF initiation codon (Fig. IS200.74 c). A gel shift assay demonstrated that the two regions can interact to give a slow mobility complex whether the invF RNA or tnpA (nt 1-173) were labelled. This did not occur when using tnpA RNA mutant LS (black in Fig. IS200.74 c).
Pb2+ footprinting with P32-labelled invF RNA and unlabeled WT or LS tnpA RNA revealed substantial pairing at nts 17-23 in the case of WT tnpA RNA (red inFig. IS200.74 c). This interaction was probed in vitro using an overexpressing chromosomal tnpA RNA with and without a T1 mutation which eliminates the interactions observed using Pb2+ (black in Fig. IS200.74 c). SL13344 WT RNA from late exponential phase showed reduced invF and sicA RNA levels while the T1 mutation had no effect.
It not yet clear whether one or both processed tnpA small RNAs base-pair with invF mRNA to inhibit expression and induce a rapid transcript turnover neither is the role of art200 in tnpA regulation of SPI-1 gene expression [153].
Both tnpA RNA processing sites (Fig. IS200.74 a) nts 19 (A) and 108 (B) are located approximately at the boundaries of the art200 pairing sequence (Fig. IS200.74 d) raising the possibility that art200 might in some way be involved in tnpA RNA processing. There is a loose correlation of growth phase dependent expression of art200 and SPI-1 genes consistent with the notion that art200 might “silence” tnpA RNA to liberate invF expression [153].
Growth phase dependence.
InvF expression increases in late exponential/early stationary phase [150] and it is possible that this is the result of changes in tnpA RNA levels. To examine this, expression of genes influenced by tnpA RNA (invF, sicA, sipB, sipC and prgH) were monitored during different growth phases in WT and in the tnpA RNA over-expressing strain, both of which had identical growth rates. TnpA RNA over-expression had no effect during lag- or early exponential-phase but in late-exponential phase, tnpA RNA over-expression reduced invF (2-fold), sicA (5.5-fold), sipB (4-fold) and sipC (2fold) but not the invF-independent SPI-1 encoded prgH.
Overall, the data indicated that tnpA RNA over-expression affects invF RNA levels only when expressed at lower levels than invF RNA, implying a stoichiometry between both transcripts and a direct tnpA-invF RNA interaction. Moreover, tnpA RNA over-expression only affected SPI-1 gene expression in early-exponential phase and late-exponential where the WT tnpA RNA levels are limiting relative to invF RNA.
These results suggest that the native IS200 copies may be important in controlling expression of the pathogenicity island. This was tested by comparing invF RNA expression following deletion of 4 of the 7 IS200 copies (Δ tnpA4/7) where, in both early- and late-exponential phase, native tnpA RNA was reduced 2.5 fold and invF RNA increased 2 fold in early- and 1.5 fold in late-exponential phase. Additionally, excising all 7 IS200 copies (Δ tnpA7/7), resulted in a reduction in growth rate and a 25 fold increase in SPI-1 expression. These effects were reversed on introducing a chromosomal module (tnpA 7::kan-pTet, in which a kan pTet cassette was placed in front of tnpA 7 – in IS200#7- such that the Tet promoter drives transcription of tnpA) which overexpresses tnpA RNA. Additionally, in the HeLa cell invasion assay, the overexpressing tnpA strain showed reduced invasiveness while the Δ tnpA7/7 strain was between 5 and 10 fold more invasive.
TnpA controls expression of more than 200 host genes
Further analysis of differential gene expression [170] using comparative RNA seq between the invasive Salmonella SL1344 and the derivative strain lackng all seven IS200 copies revealed more than 200 genes affected by the tnpA 5’UTR. These included master regulators for HilD (invasion) and FlhDC (flagellar) regulons, the cysteine biosynthesis regulon and phsABC, a thiosulfate reductase operon. These effects resulted in an 80-fold increase in a HeLa cell invasion assay. Some of these interactions are shown in Fig. IS200.75.

These genes are central to Salmonella virulence: The HilD transcription factor is a central control element in a complex cascade of reactions. It acts upstream of IlvF. The phsABC operon is involved in anerobic growth conditions and its inactivation results in increased invasiveness. Other details can be found in Trussler et al 2024[170] and Lou et al [171].
A model of the IS200 regulatory network
A model for RNA-mediated metabolism involving IS200 RNA (Fig. IS200.76; [153]) proposes that the 5’- UTR terminal segment of tnpA mRNA assumes a folded structure recognized by art200 asRNA facilitated by Hfq which blocks TnpA translation and leadsto degradation (bottom) or is processed and recognizes the 5’ end of the invF message blocking InvF translation and provoking degradation. Thus deletion of native IS200 copies increases invasion (and reduces growth rate) [153]. One possibility is that art200 pairs with the tnpA 5’UTR to prevent its processing.

TnpB co-option as transcription factors.
The notion that RuvC evolved to generate the TnpB and IscB families of guide endonucleases, which maintain copy number of their associated transposable elements, and then into Cas12 and Cas9 proteins (TnpB; Fig.IS200.37), which act in bacterial immunity to invading mobile elements, led to the question of whether they might have evolved to fulfill other functions. For example, type V-K CRISPR-associated transposases similarly rely on nuclease-inactivated Cas12k homologues that are still active for RNA-guided DNA binding, facilitated programmable sequence-specific targeted transposition.
In view of the identification of atypical Cas12 homologues, Cas12c and Cas12m, which have lost their cleavage functions but not their binding function and are capable of repressing gene transcription, preventing bacteriophage proliferation [172] or plasmid invasion [173].Weigand et al., [174][175] sought to determine whether some members of the TnpB group had also been domesticated and had assumed transposition-independent functions.
Repurposing TnpB proteins
Truncated copies of TnpB had been noted early in the identification of the IS200/IS605 family and using a sample of only 85 IS605 derivatives in ISfinder. They had been believed to be decay products but with hindsight should be considered as repurposed derivatives (IS Decay; Fig. IS200.30; see [176][177]. Weigand et al.,[174][175] used a library of nearly 96,000 TnpB-related proteins extracted from public databases. They identified over 500 nuclease-inactive variants containing at least 2 mutations in the DED catalytic nuclease triad. Doubly inactivated catalytic mutants were chosen since it had been shown that one of the three RuvC catalytic amino acids can occur at an alternative position [178]. These were obtained from “diverse genetic neighborhoods” including examples which were not associated with tnpA.
In view of their distribution across the phylogenetic tree (Fig. IS200.77), Weigand et al., [174][175] suggest that they may have arisen independently over time from different tnpB genes. These showed different degrees of mutation ranging from examples with one or more mutated catalytic site residues to homologues with C-terminal truncated domains removing RuvC and the zinc finger domains (Fig. IS200.77). Interestingly, among the mutated TnpB examples in the original ISfinder sample, 2 had lost their C-terminal zinc finger domains [177] and, since they remain associated with a tnpA gene, might represent examples of a tnpB on an evolutionary path to alternative functions.

Weigand et al., [174][175] also used AlphaFold predictions which provided supporting structural evidence of sequential mutation of the TnpB nuclease catalytic site. However, in each case, the TnpB RNA-binding interface, which determines TnpB DNA targeting functions, had been retained.
Further studies revealed that several of these TnpB derivatives with inactive nucleases function as repressors of expression of a number of genes: they were called TldRs (for TnpB-Like nuclease- Dead Repressors).

TldR Genetic Context
Many TldR were found neighboring non-IS-related genes. These were found to be frequently clade-specific (Fig. IS200.77): one group was consistently associated with ABC transporter systems genes including oppF, mainly present in Enterococci and located downstream (Fig. IS200.79A b); another with fliC, encoding the flagellin subunit of flagellar assemblies in Enterobacteriaceae and associated with a prophage (called fliCp to distinguish it from the genomic copy – note that the fliCp-associated TldR was identified in nearly 30 prophages), also located downstream; and a third group from Clostridia also associated with flagellin genes and with the carbon storage regulator, csrA, involved in flagellar subunit regulation and generally located downstream. Such strong associations suggested the TldRs may have functional role.
TldR Guide RNA Identification and binding
It was of considerable interest to determine whether small guide RNAs (gRNA) are associated with TldRs. Generally, these are composed of a “scaffold” domain followed by a guide sequence produced from the flanking sequence at the right end (RE) of the IS (see: Structure of TnpB-reRNA in association with DNA). However, since there are no RE associated with the TldRs to define potential guide sequences, Weigand et al., [174][175] used a co-variance approach previously used for gRNA identification (see: Conserved secondary structure motifs; [179] combined with BLAST. This identified the LE/RE boundaries and potential guide RNAs associated with active TnpB homologues closely related to fliC-associated and oppF-associated TldRs (Fig. IS200.79A c) and from these, they deduced the potential gRNA sequences of the fliC-associated and oppF-associated TldRs. In addition, RNA-seq datasets from Enterococci carrying fliC–tldR or oppF–tldR [180] revealed reads covering the TldR orfs and the proposed RNA predicted from the co-variance, thus confirming their expression.
TldR gRNA expression was investigated by cloning and expressing Enterococcal FLAG-tagged fliCP-associated TldR (Enterobacter hormaechei, EhoTldR; Fig. IS200.79 A anf B) and oppF-associated TldR (Entercoccus faecalis, Efa1TldR) on a 240 bp DNA segment including their putative guide RNA scaffold and 20-bp guide sequence in E.coli. RNA immunoprecipitation (IP) on total RNA analyzed by sequencing and mapping, revealed an 113nt EhoTldR gRNA comprising a 97 nt scaffold and a downstream 16 nt guide sequence and a 109nt Efa1TldR gRNA, comprising a 100-nt scaffold and an approximately 9-nt guide. A shorter guide of 11nt was also identified from a homologue in publicly available RNA-seq data [180].
Although TnpB has been shown to process its transcript to generate the final gRNA (see: Generating re(ω)RNA: Processing; [181] using its RuvC activity, the TldR are RuvC-defective. It was suggested that the mature gRNA may simply be a structure which is protected from other cell ribonucleases.

What do TldR gRNA target?
To determine the targets of fliCP- phage-associated TldR, a library of gRNA assembled from bioinformatic and R IP-seq analyses [174][175] was used as queries in a BLAST search. This yielded a strong match of the prophage –associated in a genomic region carrying flagellar genes from E. cloacae AR_1054 (Fig. IS200.80a) and located between the genomic fliC and fliD genes (Fig.IS200.80a top) at a position distinct from the prophage.
The putative gRNA target overlapped a potential σ28 promoter (Fig.IS200.80a middle) which, in E. coli, is recognized by FilA/σ28 and drives fil expression. Moreover, the putative target sequence was flanked on its 5’ side by the sequence GTTAT. This is conserved in a number of prophage genomes identified in several Enterobacter species (Fig.IS200.80a bottom) and resembles the TAM sequences recognized by active TnpB nucleases similar to the TldRs.
It was suggested that phage TldR gRNA might repress expression of the host FliC while maintaining its own FliCp synthesis.
Further studies used the short 9nt guide associated with oppF TldRs together with the TAM sequence, TTTAA/TTTAT, of related TnpB and uncovered a potential target upstream of the initiation codon of a chromosomal oppA ABC transporter gene in E. faecalis (TAM sequence, TTAAA; Fig.IS200.80b) and E. cecorum (TAM sequence, TTTAA; Fig.IS200.80c). In both cases, the gRNA sequence (9nt for E. faecalis and 7nt E. cecorum) was complementary to sequences overlapping the promoter, again suggesting that the TldR/gRNA would repress expression of the associated opp operon by competition with RNA polymerase. Interestingly the analysis of E. cecorum identified a significant number of additional potential targets (Fig.IS200.81), all with a 7nt complementary core and a 5’TTTAA TAM sequence. This suggests that the oppF-TldR may be involved in an extended regulatory network.

Functional Analysis: TldR/gRNA target their cognate target sites
Fifteen TldR/gRNA examples were chosen for functional analysis: several fliCP-TldR (Fig. IS200.78B top) and oppF-associated (Fig. IS200.78B bottom) loci were cloned together with their putative gRNA and expressed in an E. coli derivative carrying predicted target site integrated into the chromosome. Their genome-wide binding specificity was then ascertained by CHIP-Seq (chromatin immunoprecipitation) using FLAG-tagged TnpB and TldR and subsequently sequenced (ChIP–seq). For the majority of the examples, the results revealed strong peaks corresponding to the expected target site: the nuclease-inactive TldR therefore retained the ability to bind to specific target sites in genomic DNA.
Functional Analysis: extent of target complementarity required for TldR/gRNA binding.
The results also included a significant proportion of “off-target” sites. When analyzed in more detail, 3 prominent off-target peaks were observed for the fliC-associated TldR homologues: Kpi, Eco, Eko1, Eko2 and Eho. One of these was found to be the E. coli host chromosomal filC and filD intergenic region which differs from the Enterobacter cloacae sp. AR_15 fliC-TldR, by 5 of the core complementary nucleotides (Fig. IS200. 80a; Fig. IS200.82). A similar analysis of off-target oppF-associated TldR insertions (Eca-, Emu, Efa, Tos and Ece) (Fig. IS200.82) also indicated a rather relaxed recognition. These data are consistent with the approximately 6ny “seed” sequence found to be sufficient for certain Cas12a homologues [182] and corresponds to the length of the core sequence complementarity found for the multiple potential TAM-associated EceTldR targets identified in the E. cecorum genome (Fig. IS200.81).
Systematic analysis of all CHIP-seq peaks for enriched motifs (see [179]) revealed that fliCp-associated TldRs enriched for GTTAT identical to that flanking fliC promoters (Fig.IS200.80a), while oppF-associated TldR homologues enriched TTTAA motifs, the TAM specificity predicted for closely related TnpB relatives (TTTAA and TTTAT) (Fig.IS200.80b)

Functional Analysis: TldR are inactive in nuclease functions.
To determine that the TldR derivatives identified in the study were truly nuclease-deficient, they were tested, together with the related active TnpB derivatives using a plasmid interference assay (Fig.IS200.54a; TnpBGst and IscBGst proteins are active RNA-guided Nucleases; [179]). All 4 FliC TldR-related nuclease proficient TnpB homologues (Fig. IS200.79B top), reduced the CFU (colony forming units) in this assay whereas there was no effect with the 7 TdlR proteins (Fig. IS200.79B top) and all 4 oppF TldR-related nuclease proficient TnpB homologues (Fig. IS200.79B bottom), reduced the CFU whereas there was no effect with the 8 opp7 TdlR proteins (Fig. IS200.79B bottom).
It was concluded therefore that the TldRs are RNA-guided DNA proteins without nuclease activity [174][175].

Functional Analysis: target DNA binding by TldR modulates gene expression.
To determine whether the Tld systems modulate gene expression by target binding, [174][175] used an RFP/GFP assay in which gfp chromosomal expression would act as a standard control while TldR binding would be expected to repress rfp expression (Fig. IS200.83). The assay involved two plasmids: one which supplies gRNA and the TldR and another which carries the target sequence upstream of the rfp gene. gRNAs were designed to target promoter sequences occluding transcription initiation by or to target the 5′ UTR to block transcription elongation.
Using promoter targeting gRNAs, fliCp(Eho)- and oppF(Efa1)-associated TldR strongly repressed RFP when targeting the sense (top) strand. This is the native target orientation in the fliCp promoter (Fig. IS200.83 top). Shorter stretches of complementarity between target and gRNA were tested and a 6nt sequence showed repression similar to but a little lower than the 20nt guide sequence. Removal of the short sequence 5’ to the guide had little effect (Fig.IS200.83 bottom).
Repression was largely unaffected when the target was placed in the 5’UTR (i.e. downstream of the promoter) on the top strand. When placed on the bottom strand some repression could be detected for a small subgroup of both fliC- and oppF-associated TdlR.
Thus nuclease deficient TldR can efficiently repress downstream genes in a position- and orientation-dependent way.

FliCp-TldR from prophage helps supplant host FliC in flagella structures.
Inspection of the phage and bacterial FliC structures indicated that although the surface-exposed structures were very different, the protomer-protomer interface surface was well conserved suggesting that the phage and host proteins are interchangeable in flagella assembly. To test the notion that prophage FliCp replaces the host FliC allowing the phage to assume control of host flagella composition via TldR host gene repression, total RNA-seq of three FliC-Tdlr carrying lysogenic strains and one which is devoid of the prophage was undertaken.
This demonstrated that strong expression of gRNA with the expected 5’ and 3’ boundaries occurred in the fliCP-associated TldR carrying strains. In these strains, expression of host fliC compared to that of the host fliD was nearly undetectable whereas the phage fliCP gene was strongly expressed. In the prophage-free strain the host fliC was strongly expressed. That this effect was due to TldR repression, strains deleted for tldR, tldR–gRNA, the entire fliCP–TldR–gRNA and the entire prophage were created. All led to about a 100-fold increase in host fliC. Additionally, substitution of the guide segment of the gRNA for a non-targeting sequence had the same effect. Moreover, the de-repression of the host fliC gene could be reversed in the tldR gRNA deletion mutant by trans-complementation introducing a plasmid-encoded filCP-TdlR/gRNA cassette.
The data therefore show that host flagella by a coupled host fliC repression and increased incorporation of the phage FliCP product into the host flagella.
It will now be of interest to examine the impact of other TdlR on their bacterial host.
Acknowledgements
We are grateful to Fred Dyda and Alison Hickman for advice concerning transposition mechanism, to Orsyla Barabas for certain figures and videos of structures, and to Kira Makarova, Virginijus Šikšnys, and Sam Sternberg for advice concerning the RNA guide endonucleases. The Siksnys group also kindly supplied the Cas12 structural panel. Thanks also to David Haniford for comments on the impact of IS200 on expression of the SPI-1 Salmonella virulence genes. We are also grateful to all the above for permission permission to use derivatives of their published figures
Bibliography
- ↑ 1.0 1.1 1.2 1.3 1.4 Lam S, Roth JR . IS200: a Salmonella-specific insertion sequence. - Cell: 1983 Oct, 34(3);951-60 [PubMed:6313217] [DOI]
- ↑ 2.0 2.1 2.2 2.3 2.4 Beuzón CR, Chessa D, Casadesús J . IS200: an old and still bacterial transposon. - Int Microbiol: 2004 Mar, 7(1);3-12 [PubMed:15179601]
- ↑ 3.0 3.1 Lam S, Roth JR . Genetic mapping of IS200 copies in Salmonella typhimurim strain LT2. - Genetics: 1983 Dec, 105(4);801-11 [PubMed:6315530] [DOI]
- ↑ 4.0 4.1 4.2 Lam S, Roth JR . Structural and functional studies of insertion element IS200. - J Mol Biol: 1986 Jan 20, 187(2);157-67 [PubMed:3009825] [DOI]
- ↑ 5.0 5.1 Beuzón CR, Casadesús J . Conserved structure of IS200 elements in Salmonella. - Nucleic Acids Res: 1997 Apr 1, 25(7);1355-61 [PubMed:9060429] [DOI]
- ↑ Casadesus J, Roth JR . Absence of insertions among spontaneous mutants of Salmonella typhimurium. - Mol Gen Genet: 1989 Apr, 216(2-3);210-6 [PubMed:2546038] [DOI]
- ↑ Haack KR, Roth JR . Recombination between chromosomal IS200 elements supports frequent duplication formation in Salmonella typhimurium. - Genetics: 1995 Dec, 141(4);1245-52 [PubMed:8601470] [DOI]
- ↑ 8.0 8.1 Murai N, Kamata H, Nagashima Y, Yagisawa H, Hirata H . A novel insertion sequence (IS)-like element of the thermophilic bacterium PS3 promotes expression of the alanine carrier protein-encoding gene. - Gene: 1995 Sep 22, 163(1);103-7 [PubMed:7557457] [DOI]
- ↑ 9.0 9.1 9.2 Bancroft I, Wolk CP . Characterization of an insertion sequence (IS891) of novel structure from the cyanobacterium Anabaena sp. strain M-131. - J Bacteriol: 1989 Nov, 171(11);5949-54 [PubMed:2553665] [DOI]
- ↑ Donadio S, Staver MJ . IS1136, an insertion element in the erythromycin gene cluster of Saccharopolyspora erythraea. - Gene: 1993 Apr 15, 126(1);147-51 [PubMed:8386127] [DOI]
- ↑ 11.0 11.1 11.2 11.3 11.4 Kersulyte D, Akopyants NS, Clifton SW, Roe BA, Berg DE . Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. - Gene: 1998 Nov 26, 223(1-2);175-86 [PubMed:9858724] [DOI]
- ↑ 12.0 12.1 12.2 Kersulyte D, Mukhopadhyay AK, Shirai M, Nakazawa T, Berg DE . Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. - J Bacteriol: 2000 Oct, 182(19);5300-8 [PubMed:10986230] [DOI]
- ↑ Gordon SV, Heym B, Parkhill J, Barrell B, Cole ST . New insertion sequences and a novel repeated sequence in the genome of Mycobacterium tuberculosis H37Rv. - Microbiology (Reading): 1999 Apr, 145 ( Pt 4);881-892 [PubMed:10220167] [DOI]
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Kersulyte D, Velapatiño B, Dailide G, Mukhopadhyay AK, Ito Y, Cahuayme L, Parkinson AJ, Gilman RH, Berg DE . Transposable element ISHp608 of Helicobacter pylori: nonrandom geographic distribution, functional organization, and insertion specificity. - J Bacteriol: 2002 Feb, 184(4);992-1002 [PubMed:11807059] [DOI]
- ↑ 15.0 15.1 15.2 Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M . Everyman's Guide to Bacterial Insertion Sequences. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0030-2014 [PubMed:26104715] [DOI]
- ↑ Thomas J, Pritham EJ . Helitrons, the Eukaryotic Rolling-circle Transposable Elements. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350323] [DOI]
- ↑ 17.0 17.1 Chandler M, de la Cruz F, Dyda F, Hickman AB, Moncalian G, Ton-Hoang B . Breaking and joining single-stranded DNA: the HUH endonuclease superfamily. - Nat Rev Microbiol: 2013 Aug, 11(8);525-38 [PubMed:23832240] [DOI]
- ↑ Höök-Nikanne J, Berg DE, Peek RM Jr, Kersulyte D, Tummuru MK, Blaser MJ . DNA sequence conservation and diversity in transposable element IS605 of Helicobacter pylori. - Helicobacter: 1998 Jun, 3(2);79-85 [PubMed:9631304] [DOI]
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 Ronning DR, Guynet C, Ton-Hoang B, Perez ZN, Ghirlando R, Chandler M, Dyda F . Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. - Mol Cell: 2005 Oct 7, 20(1);143-54 [PubMed:16209952] [DOI]
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Ton-Hoang B, Guynet C, Ronning DR, Cointin-Marty B, Dyda F, Chandler M . Transposition of ISHp608, member of an unusual family of bacterial insertion sequences. - EMBO J: 2005 Sep 21, 24(18);3325-38 [PubMed:16163392] [DOI]
- ↑ 21.0 21.1 21.2 21.3 21.4 Guynet C, Hickman AB, Barabas O, Dyda F, Chandler M, Ton-Hoang B . In vitro reconstitution of a single-stranded transposition mechanism of IS608. - Mol Cell: 2008 Feb 15, 29(3);302-12 [PubMed:18280236] [DOI]
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 22.8 Barabas O, Ronning DR, Guynet C, Hickman AB, Ton-Hoang B, Chandler M, Dyda F . Mechanism of IS200/IS605 family DNA transposases: activation and transposon-directed target site selection. - Cell: 2008 Jan 25, 132(2);208-20 [PubMed:18243097] [DOI]
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 Pasternak C, Ton-Hoang B, Coste G, Bailone A, Chandler M, Sommer S . Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. - PLoS Genet: 2010 Jan 15, 6(1);e1000799 [PubMed:20090938] [DOI]
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 Ton-Hoang B, Pasternak C, Siguier P, Guynet C, Hickman AB, Dyda F, Sommer S, Chandler M . Single-stranded DNA transposition is coupled to host replication. - Cell: 2010 Aug 6, 142(3);398-408 [PubMed:20691900] [DOI]
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 25.7 25.8 Hickman AB, James JA, Barabas O, Pasternak C, Ton-Hoang B, Chandler M, Sommer S, Dyda F . DNA recognition and the precleavage state during single-stranded DNA transposition in D. radiodurans. - EMBO J: 2010 Nov 17, 29(22);3840-52 [PubMed:20890269] [DOI]
- ↑ 26.0 26.1 Filée J, Siguier P, Chandler M . Insertion sequence diversity in archaea. - Microbiol Mol Biol Rev: 2007 Mar, 71(1);121-57 [PubMed:17347521] [DOI]
- ↑ Devalckenaere A, Odaert M, Trieu-Cuot P, Simonet M . Characterization of IS1541-like elements in Yersinia enterocolitica and Yersinia pseudotuberculosis. - FEMS Microbiol Lett: 1999 Jul 1, 176(1);229-33 [PubMed:10418150] [DOI]
- ↑ Bisercić M, Ochman H . The ancestry of insertion sequences common to Escherichia coli and Salmonella typhimurium. - J Bacteriol: 1993 Dec, 175(24);7863-8 [PubMed:8253675] [DOI]
- ↑ Bisercić M, Ochman H . Natural populations of Escherichia coli and Salmonella typhimurium harbor the same classes of insertion sequences. - Genetics: 1993 Mar, 133(3);449-54 [PubMed:8384142] [DOI]
- ↑ 30.0 30.1 Beuzón CR, Marqués S, Casadesús J . Repression of IS200 transposase synthesis by RNA secondary structures. - Nucleic Acids Res: 1999 Sep 15, 27(18);3690-5 [PubMed:10471738] [DOI]
- ↑ Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, Hinton JC, Vogel J . Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. - PLoS Genet: 2008 Aug 22, 4(8);e1000163 [PubMed:18725932] [DOI]
- ↑ Ellis MJ, Trussler RS, Haniford DB . A cis-encoded sRNA, Hfq and mRNA secondary structure act independently to suppress IS200 transposition. - Nucleic Acids Res: 2015 Jul 27, 43(13);6511-27 [PubMed:26044710] [DOI]
- ↑ Odaert M, Devalckenaere A, Trieu-Cuot P, Simonet M . Molecular characterization of IS1541 insertions in the genome of Yersinia pestis. - J Bacteriol: 1998 Jan, 180(1);178-81 [PubMed:9422611] [DOI]
- ↑ 34.0 34.1 34.2 Boocock MR, Rice PA . A proposed mechanism for IS607-family serine transposases. - Mob DNA: 2013 Nov 6, 4(1);24 [PubMed:24195768] [DOI]
- ↑ Akopyants NS, Fradkov A, Diatchenko L, Hill JE, Siebert PD, Lukyanov SA, Sverdlov ED, Berg DE . PCR-based subtractive hybridization and differences in gene content among strains of Helicobacter pylori. - Proc Natl Acad Sci U S A: 1998 Oct 27, 95(22);13108-13 [PubMed:9789049] [DOI]
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 Xiang G, Li Y, Sun J, Huo Y, Cao S, Cao Y, Guo Y, Yang L, Cai Y, Zhang YE, Wang H . Evolutionary mining and functional characterization of TnpB nucleases identify efficient miniature genome editors. - Nat Biotechnol: 2024 May, 42(5);745-757 [PubMed:37386294] [DOI]
- ↑ 37.0 37.1 Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, Butterfield CN, Hernsdorf AW, Amano Y, Ise K, Suzuki Y, Dudek N, Relman DA, Finstad KM, Amundson R, Thomas BC, Banfield JF . A new view of the tree of life. - Nat Microbiol: 2016 Apr 11, 1;16048 [PubMed:27572647] [DOI]
- ↑ Islam SM, Hua Y, Ohba H, Satoh K, Kikuchi M, Yanagisawa T, Narumi I . Characterization and distribution of IS8301 in the radioresistant bacterium Deinococcus radiodurans. - Genes Genet Syst: 2003 Oct, 78(5);319-27 [PubMed:14676423] [DOI]
- ↑ 39.0 39.1 Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M . Reassembly of shattered chromosomes in Deinococcus radiodurans. - Nature: 2006 Oct 5, 443(7111);569-73 [PubMed:17006450] [DOI]
- ↑ Stanley TL, Ellermeier CD, Slauch JM . Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar typhimurium survival in Peyer's patches. - J Bacteriol: 2000 Aug, 182(16);4406-13 [PubMed:10913072] [DOI]
- ↑ 41.00 41.01 41.02 41.03 41.04 41.05 41.06 41.07 41.08 41.09 41.10 41.11 41.12 41.13 41.14 41.15 41.16 Meers C, Le HC, Pesari SR, Hoffmann FT, Walker MWG, Gezelle J, Tang S, Sternberg SH . Transposon-encoded nucleases use guide RNAs to promote their selfish spread. - Nature: 2023 Oct, 622(7984);863-871 [PubMed:37758954] [DOI]
- ↑ 42.00 42.01 42.02 42.03 42.04 42.05 42.06 42.07 42.08 42.09 42.10 42.11 42.12 42.13 42.14 42.15 42.16 42.17 Kapitonov VV, Makarova KS, Koonin EV . ISC, a Novel Group of Bacterial and Archaeal DNA Transposons That Encode Cas9 Homologs. - J Bacteriol: 2015 Dec 28, 198(5);797-807 [PubMed:26712934] [DOI]
- ↑ 43.00 43.01 43.02 43.03 43.04 43.05 43.06 43.07 43.08 43.09 43.10 43.11 43.12 43.13 43.14 43.15 43.16 43.17 43.18 43.19 43.20 43.21 43.22 Altae-Tran H, Kannan S, Demircioglu FE, Oshiro R, Nety SP, McKay LJ, Dlakić M, Inskeep WP, Makarova KS, Macrae RK, Koonin EV, Zhang F . The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. - Science: 2021 Oct, 374(6563);57-65 [PubMed:34591643] [DOI]
- ↑ Koonin EV, Ilyina TV . Computer-assisted dissection of rolling circle DNA replication. - Biosystems: 1993, 30(1-3);241-68 [PubMed:8374079] [DOI]
- ↑ 45.0 45.1 Lee HH, Yoon JY, Kim HS, Kang JY, Kim KH, Kim DJ, Ha JY, Mikami B, Yoon HJ, Suh SW . Crystal structure of a metal ion-bound IS200 transposase. - J Biol Chem: 2006 Feb 17, 281(7);4261-6 [PubMed:16340015] [DOI]
- ↑ 46.0 46.1 46.2 46.3 He S, Guynet C, Siguier P, Hickman AB, Dyda F, Chandler M, Ton-Hoang B . IS200/IS605 family single-strand transposition: mechanism of IS608 strand transfer. - Nucleic Acids Res: 2013 Mar 1, 41(5);3302-13 [PubMed:23345619] [DOI]
- ↑ 47.0 47.1 47.2 47.3 47.4 47.5 47.6 47.7 He S, Hickman AB, Dyda F, Johnson NP, Chandler M, Ton-Hoang B . Reconstitution of a functional IS608 single-strand transpososome: role of non-canonical base pairing. - Nucleic Acids Res: 2011 Oct, 39(19);8503-12 [PubMed:21745812] [DOI]
- ↑ 48.0 48.1 48.2 48.3 48.4 Guynet C, Achard A, Hoang BT, Barabas O, Hickman AB, Dyda F, Chandler M . Resetting the site: redirecting integration of an insertion sequence in a predictable way. - Mol Cell: 2009 Jun 12, 34(5);612-9 [PubMed:19524540] [DOI]
- ↑ Morero NR, Zuliani C, Kumar B, Bebel A, Okamoto S, Guynet C, Hickman AB, Chandler M, Dyda F, Barabas O . Targeting IS608 transposon integration to highly specific sequences by structure-based transposon engineering. - Nucleic Acids Res: 2018 May 4, 46(8);4152-4163 [PubMed:29635476] [DOI]
- ↑ 50.0 50.1 Mennecier S, Servant P, Coste G, Bailone A, Sommer S . Mutagenesis via IS transposition in Deinococcus radiodurans. - Mol Microbiol: 2006 Jan, 59(1);317-25 [PubMed:16359337] [DOI]
- ↑ Parks AR, Li Z, Shi Q, Owens RM, Jin MM, Peters JE . Transposition into replicating DNA occurs through interaction with the processivity factor. - Cell: 2009 Aug 21, 138(4);685-95 [PubMed:19703395] [DOI]
- ↑ Hu WY, Derbyshire KM . Target choice and orientation preference of the insertion sequence IS903. - J Bacteriol: 1998 Jun, 180(12);3039-48 [PubMed:9620951] [DOI]
- ↑ Roberts D, Hoopes BC, McClure WR, Kleckner N . IS10 transposition is regulated by DNA adenine methylation. - Cell: 1985 Nov, 43(1);117-30 [PubMed:3000598] [DOI]
- ↑ Yin JC, Krebs MP, Reznikoff WS . Effect of dam methylation on Tn5 transposition. - J Mol Biol: 1988 Jan 5, 199(1);35-45 [PubMed:2451025] [DOI]
- ↑ Dodson KW, Berg DE . Factors affecting transposition activity of IS50 and Tn5 ends. - Gene: 1989, 76(2);207-13 [PubMed:2546858] [DOI]
- ↑ Spradling AC, Bellen HJ, Hoskins RA . Drosophila P elements preferentially transpose to replication origins. - Proc Natl Acad Sci U S A: 2011 Sep 20, 108(38);15948-53 [PubMed:21896744] [DOI]
- ↑ Zechner EL, Wu CA, Marians KJ . Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. III. A polymerase-primase interaction governs primer size. - J Biol Chem: 1992 Feb 25, 267(6);4054-63 [PubMed:1531480]
- ↑ Wu CA, Zechner EL, Reems JA, McHenry CS, Marians KJ . Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. V. Primase action regulates the cycle of Okazaki fragment synthesis. - J Biol Chem: 1992 Feb 25, 267(6);4074-83 [PubMed:1740453]
- ↑ Lau IF, Filipe SR, Søballe B, Økstad OA, Barre FX, Sherratt DJ . Spatial and temporal organization of replicating Escherichia coli chromosomes. - Mol Microbiol: 2003 Aug, 49(3);731-43 [PubMed:12864855] [DOI]
- ↑ Lavatine L, He S, Caumont-Sarcos A, Guynet C, Marty B, Chandler M, Ton-Hoang B . Single strand transposition at the host replication fork. - Nucleic Acids Res: 2016 Sep 19, 44(16);7866-83 [PubMed:27466393] [DOI]
- ↑ Hansen MT . Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. - J Bacteriol: 1978 Apr, 134(1);71-5 [PubMed:649572] [DOI]
- ↑ Harsojo, Kitayama S, Matsuyama A . Genome multiplicity and radiation resistance in Micrococcus radiodurans. - J Biochem: 1981 Sep, 90(3);877-80 [PubMed:7309705] [DOI]
- ↑ Kim NH, Lee G, Sherer NA, Martini KM, Goldenfeld N, Kuhlman TE . Real-time transposable element activity in individual live cells. - Proc Natl Acad Sci U S A: 2016 Jun 28, 113(26);7278-83 [PubMed:27298350] [DOI]
- ↑ Markwardt ML, Kremers GJ, Kraft CA, Ray K, Cranfill PJ, Wilson KA, Day RN, Wachter RM, Davidson MW, Rizzo MA . An improved cerulean fluorescent protein with enhanced brightness and reduced reversible photoswitching. - PLoS One: 2011 Mar 29, 6(3);e17896 [PubMed:21479270] [DOI]
- ↑ Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A . A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. - Nat Biotechnol: 2002 Jan, 20(1);87-90 [PubMed:11753368] [DOI]
- ↑ Shapiro JA, Higgins NP . Variation of beta-galactosidase expression from Mudlac elements during the development of Escherichia coli colonies. - Ann Inst Pasteur Microbiol: 1988 Jan-Feb, 139(1);79-103 [PubMed:2838063] [DOI]
- ↑ Shapiro JA, Higgins NP . Differential activity of a transposable element in Escherichia coli colonies. - J Bacteriol: 1989 Nov, 171(11);5975-86 [PubMed:2553666] [DOI]
- ↑ 68.0 68.1 68.2 Pasternak C, Dulermo R, Ton-Hoang B, Debuchy R, Siguier P, Coste G, Chandler M, Sommer S . ISDra2 transposition in Deinococcus radiodurans is downregulated by TnpB. - Mol Microbiol: 2013 Apr, 88(2);443-55 [PubMed:23461641] [DOI]
- ↑ Chylinski K, Makarova KS, Charpentier E, Koonin EV . Classification and evolution of type II CRISPR-Cas systems. - Nucleic Acids Res: 2014 Jun, 42(10);6091-105 [PubMed:24728998] [DOI]
- ↑ Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, Abudayyeh OO, Gootenberg JS, Makarova KS, Wolf YI, Severinov K, Zhang F, Koonin EV . Diversity and evolution of class 2 CRISPR-Cas systems. - Nat Rev Microbiol: 2017 Mar, 15(3);169-182 [PubMed:28111461] [DOI]
- ↑ Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, Moineau S, Mojica FJM, Scott D, Shah SA, Siksnys V, Terns MP, Venclovas Č, White MF, Yakunin AF, Yan W, Zhang F, Garrett RA, Backofen R, van der Oost J, Barrangou R, Koonin EV . Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. - Nat Rev Microbiol: 2020 Feb, 18(2);67-83 [PubMed:31857715] [DOI]
- ↑ 72.00 72.01 72.02 72.03 72.04 72.05 72.06 72.07 72.08 72.09 72.10 72.11 72.12 72.13 72.14 72.15 72.16 72.17 72.18 72.19 72.20 72.21 72.22 72.23 72.24 72.25 72.26 72.27 72.28 Karvelis T, Druteika G, Bigelyte G, Budre K, Zedaveinyte R, Silanskas A, Kazlauskas D, Venclovas Č, Siksnys V . Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. - Nature: 2021 Nov, 599(7886);692-696 [PubMed:34619744] [DOI]
- ↑ 73.0 73.1 Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA . Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. - Science: 2014 Mar 14, 343(6176);1247997 [PubMed:24505130] [DOI]
- ↑ 74.0 74.1 Gasiunas G, Barrangou R, Horvath P, Siksnys V . Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. - Proc Natl Acad Sci U S A: 2012 Sep 25, 109(39);E2579-86 [PubMed:22949671] [DOI]
- ↑ Makarova KS, Aravind L, Wolf YI, Koonin EV . Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. - Biol Direct: 2011 Jul 14, 6;38 [PubMed:21756346] [DOI]
- ↑ Swarts DC . Stirring Up the Type V Alphabet Soup. - CRISPR J: 2019 Feb, 2;14-16 [PubMed:31021231] [DOI]
- ↑ Xiao R, Li Z, Wang S, Han R, Chang L . Structural basis for substrate recognition and cleavage by the dimerization-dependent CRISPR-Cas12f nuclease. - Nucleic Acids Res: 2021 Apr 19, 49(7);4120-4128 [PubMed:33764415] [DOI]
- ↑ 78.0 78.1 Takeda SN, Nakagawa R, Okazaki S, Hirano H, Kobayashi K, Kusakizako T, Nishizawa T, Yamashita K, Nishimasu H, Nureki O . Structure of the miniature type V-F CRISPR-Cas effector enzyme. - Mol Cell: 2021 Feb 4, 81(3);558-570.e3 [PubMed:33333018] [DOI]
- ↑ 79.00 79.01 79.02 79.03 79.04 79.05 79.06 79.07 79.08 79.09 79.10 79.11 79.12 79.13 79.14 79.15 79.16 79.17 Yoon PH, Skopintsev P, Shi H, Chen L, Adler BA, Al-Shimary M, Craig RJ, Loi KJ, DeTurk EC, Li Z, Amerasekera J, Trinidad M, Nisonoff H, Chen K, Lahiri A, Boger R, Jacobsen S, Banfield JF, Doudna JA . Eukaryotic RNA-guided endonucleases evolved from a unique clade of bacterial enzymes. - Nucleic Acids Res: 2023 Dec 11, 51(22);12414-12427 [PubMed:37971304] [DOI]
- ↑ 80.0 80.1 80.2 80.3 80.4 80.5 80.6 80.7 80.8 80.9 Bao W, Jurka J . Homologues of bacterial TnpB_IS605 are widespread in diverse eukaryotic transposable elements. - Mob DNA: 2013 Apr 1, 4(1);12 [PubMed:23548000] [DOI]
- ↑ Rex G, Surin B, Besse G, Schneppe B, McCarthy JE . The mechanism of translational coupling in Escherichia coli. Higher order structure in the atpHA mRNA acts as a conformational switch regulating the access of de novo initiating ribosomes. - J Biol Chem: 1994 Jul 8, 269(27);18118-27 [PubMed:7517937]
- ↑ Huber M, Faure G, Laass S, Kolbe E, Seitz K, Wehrheim C, Wolf YI, Koonin EV, Soppa J . Translational coupling via termination-reinitiation in archaea and bacteria. - Nat Commun: 2019 Sep 5, 10(1);4006 [PubMed:31488843] [DOI]
- ↑ Gomes-Filho JV, Zaramela LS, Italiani VC, Baliga NS, Vêncio RZ, Koide T . Sense overlapping transcripts in IS1341-type transposase genes are functional non-coding RNAs in archaea. - RNA Biol: 2015, 12(5);490-500 [PubMed:25806405] [DOI]
- ↑ Zago MA, Dennis PP, Omer AD . The expanding world of small RNAs in the hyperthermophilic archaeon Sulfolobus solfataricus. - Mol Microbiol: 2005 Mar, 55(6);1812-28 [PubMed:15752202] [DOI]
- ↑ 85.0 85.1 Jäger D, Förstner KU, Sharma CM, Santangelo TJ, Reeve JN . Primary transcriptome map of the hyperthermophilic archaeon Thermococcus kodakarensis. - BMC Genomics: 2014 Aug 16, 15(1);684 [PubMed:25127548] [DOI]
- ↑ Phok K, Moisan A, Rinaldi D, Brucato N, Carpousis AJ, Gaspin C, Clouet-d'Orval B . Identification of CRISPR and riboswitch related RNAs among novel noncoding RNAs of the euryarchaeon Pyrococcus abyssi. - BMC Genomics: 2011 Jun 13, 12;312 [PubMed:21668986] [DOI]
- ↑ 87.0 87.1 87.2 87.3 Gomes-Filho JV, Zaramela LS, Italiani VC, Baliga NS, Vêncio RZ, Koide T . Sense overlapping transcripts in IS1341-type transposase genes are functional non-coding RNAs in archaea. - RNA Biol: 2015, 12(5);490-500 [PubMed:25806405] [DOI]
- ↑ 88.0 88.1 Ibrahim AGAE, Vêncio RZN, Lorenzetti APR, Koide T . Halobacterium salinarum and Haloferax volcanii Comparative Transcriptomics Reveals Conserved Transcriptional Processing Sites. - Genes (Basel): 2021 Jun 30, 12(7); [PubMed:34209065] [DOI]
- ↑ Koide T, Reiss DJ, Bare JC, Pang WL, Facciotti MT, Schmid AK, Pan M, Marzolf B, Van PT, Lo FY, Pratap A, Deutsch EW, Peterson A, Martin D, Baliga NS . Prevalence of transcription promoters within archaeal operons and coding sequences. - Mol Syst Biol: 2009, 5;285 [PubMed:19536208] [DOI]
- ↑ Jäger D, Förstner KU, Sharma CM, Santangelo TJ, Reeve JN . Primary transcriptome map of the hyperthermophilic archaeon Thermococcus kodakarensis. - BMC Genomics: 2014 Aug 16, 15(1);684 [PubMed:25127548] [DOI]
- ↑ Klein RJ, Misulovin Z, Eddy SR . Noncoding RNA genes identified in AT-rich hyperthermophiles. - Proc Natl Acad Sci U S A: 2002 May 28, 99(11);7542-7 [PubMed:12032319] [DOI]
- ↑ Been MD, Wickham GS . Self-cleaving ribozymes of hepatitis delta virus RNA. - Eur J Biochem: 1997 Aug 1, 247(3);741-53 [PubMed:9288893] [DOI]
- ↑ Ferré-D'Amaré AR, Zhou K, Doudna JA . Crystal structure of a hepatitis delta virus ribozyme. - Nature: 1998 Oct 8, 395(6702);567-74 [PubMed:9783582] [DOI]
- ↑ Karvelis T, Young JK, Siksnys V . A pipeline for characterization of novel Cas9 orthologs. - Methods Enzymol: 2019, 616;219-240 [PubMed:30691644] [DOI]
- ↑ 95.0 95.1 95.2 95.3 95.4 95.5 95.6 95.7 Nety SP, Altae-Tran H, Kannan S, Demircioglu FE, Faure G, Hirano S, Mears K, Zhang Y, Macrae RK, Zhang F . The Transposon-Encoded Protein TnpB Processes Its Own mRNA into ωRNA for Guided Nuclease Activity. - CRISPR J: 2023 Jun, 6(3);232-242 [PubMed:37272862] [DOI]
- ↑ 96.0 96.1 Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E . The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. - Nature: 2016 Apr 28, 532(7600);517-21 [PubMed:27096362] [DOI]
- ↑ Swarts DC, van der Oost J, Jinek M . Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. - Mol Cell: 2017 Apr 20, 66(2);221-233.e4 [PubMed:28431230] [DOI]
- ↑ 98.00 98.01 98.02 98.03 98.04 98.05 98.06 98.07 98.08 98.09 98.10 98.11 Nakagawa R, Hirano H, Omura SN, Nety S, Kannan S, Altae-Tran H, Yao X, Sakaguchi Y, Ohira T, Wu WY, Nakayama H, Shuto Y, Tanaka T, Sano FK, Kusakizako T, Kise Y, Itoh Y, Dohmae N, van der Oost J, Suzuki T, Zhang F, Nureki O . Cryo-EM structure of the transposon-associated TnpB enzyme. - Nature: 2023 Apr, 616(7956);390-397 [PubMed:37020030] [DOI]
- ↑ 99.00 99.01 99.02 99.03 99.04 99.05 99.06 99.07 99.08 99.09 99.10 99.11 Sasnauskas G, Tamulaitiene G, Druteika G, Carabias A, Silanskas A, Kazlauskas D, Venclovas Č, Montoya G, Karvelis T, Siksnys V . TnpB structure reveals minimal functional core of Cas12 nuclease family. - Nature: 2023 Apr, 616(7956);384-389 [PubMed:37020015] [DOI]
- ↑ Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F . Multiplex genome engineering using CRISPR/Cas systems. - Science: 2013 Feb 15, 339(6121);819-23 [PubMed:23287718] [DOI]
- ↑ Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F . Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. - Cell: 2015 Oct 22, 163(3);759-71 [PubMed:26422227] [DOI]
- ↑ 102.0 102.1 102.2 102.3 102.4 102.5 102.6 102.7 102.8 102.9 He S, Corneloup A, Guynet C, Lavatine L, Caumont-Sarcos A, Siguier P, Marty B, Dyda F, Chandler M, Ton Hoang B . The IS200/IS605 Family and "Peel and Paste" Single-strand Transposition Mechanism. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350330] [DOI]
- ↑ 103.0 103.1 Altae-Tran H, Shmakov SA, Makarova KS, Wolf YI, Kannan S, Zhang F, Koonin EV . Diversity, evolution, and classification of the RNA-guided nucleases TnpB and Cas12. - Proc Natl Acad Sci U S A: 2023 Nov 28, 120(48);e2308224120 [PubMed:37983496] [DOI]
- ↑ 104.0 104.1 104.2 104.3 104.4 Kato K, Okazaki S, Kannan S, Altae-Tran H, Esra Demircioglu F, Isayama Y, Ishikawa J, Fukuda M, Macrae RK, Nishizawa T, Makarova KS, Koonin EV, Zhang F, Nishimasu H . Structure of the IscB-ωRNA ribonucleoprotein complex, the likely ancestor of CRISPR-Cas9. - Nat Commun: 2022 Nov 7, 13(1);6719 [PubMed:36344504] [DOI]
- ↑ 105.0 105.1 105.2 105.3 Hirano S, Kappel K, Altae-Tran H, Faure G, Wilkinson ME, Kannan S, Demircioglu FE, Yan R, Shiozaki M, Yu Z, Makarova KS, Koonin EV, Macrae RK, Zhang F . Structure of the OMEGA nickase IsrB in complex with ωRNA and target DNA. - Nature: 2022 Oct, 610(7932);575-581 [PubMed:36224386] [DOI]
- ↑ Briner AE, Donohoue PD, Gomaa AA, Selle K, Slorach EM, Nye CH, Haurwitz RE, Beisel CL, May AP, Barrangou R . Guide RNA functional modules direct Cas9 activity and orthogonality. - Mol Cell: 2014 Oct 23, 56(2);333-339 [PubMed:25373540] [DOI]
- ↑ Shibata M, Nishimasu H, Kodera N, Hirano S, Ando T, Uchihashi T, Nureki O . Real-space and real-time dynamics of CRISPR-Cas9 visualized by high-speed atomic force microscopy. - Nat Commun: 2017 Nov 10, 8(1);1430 [PubMed:29127285] [DOI]
- ↑ Sternberg SH, LaFrance B, Kaplan M, Doudna JA . Conformational control of DNA target cleavage by CRISPR-Cas9. - Nature: 2015 Nov 5, 527(7576);110-3 [PubMed:26524520] [DOI]
- ↑ Weinberg Z, Perreault J, Meyer MM, Breaker RR . Exceptional structured noncoding RNAs revealed by bacterial metagenome analysis. - Nature: 2009 Dec 3, 462(7273);656-9 [PubMed:19956260] [DOI]
- ↑
Dyall-Smith ML, Pfeiffer F, Klee K, Palm P, Gross K, Schuster SC, Rampp M, Oesterhelt D . Haloquadratum walsbyi: limited diversity in a global pond. - PLoS One: 2011, 6(6);e20968 [PubMed:21701686]
[DOI]
- ↑ Cox MM . Regulation of bacterial RecA protein function. - Crit Rev Biochem Mol Biol: 2007 Jan-Feb, 42(1);41-63 [PubMed:17364684] [DOI]
- ↑ 112.0 112.1 Braun V, Mehlig M, Moos M, Rupnik M, Kalt B, Mahony DE, von Eichel-Streiber C . A chimeric ribozyme in clostridium difficile combines features of group I introns and insertion elements. - Mol Microbiol: 2000 Jun, 36(6);1447-59 [PubMed:10931294] [DOI]
- ↑
Karpathy SE, Qin X, Gioia J, Jiang H, Liu Y, Petrosino JF, Yerrapragada S, Fox GE, Haake SK, Weinstock GM, Highlander SK . Genome sequence of Fusobacterium nucleatum subspecies polymorphum - a genetically tractable fusobacterium. - PLoS One: 2007 Aug 1, 2(7);e659 [PubMed:17668047]
[DOI]
- ↑ Tourasse NJ, Helgason E, Økstad OA, Hegna IK, Kolstø AB . The Bacillus cereus group: novel aspects of population structure and genome dynamics. - J Appl Microbiol: 2006 Sep, 101(3);579-93 [PubMed:16907808] [DOI]
- ↑ Tourasse NJ, Kolstø AB . Survey of group I and group II introns in 29 sequenced genomes of the Bacillus cereus group: insights into their spread and evolution. - Nucleic Acids Res: 2008 Aug, 36(14);4529-48 [PubMed:18587153] [DOI]
- ↑ 116.0 116.1 Tourasse NJ, Stabell FB, Reiter L, Kolstø AB . Unusual group II introns in bacteria of the Bacillus cereus group. - J Bacteriol: 2005 Aug, 187(15);5437-51 [PubMed:16030238] [DOI]
- ↑ 117.00 117.01 117.02 117.03 117.04 117.05 117.06 117.07 117.08 117.09 117.10 117.11 117.12 117.13 117.14 117.15 117.16 117.17
Žedaveinytė R, Meers C, Le HC, Mortman EE, Tang S, Lampe GD, Pesari SR, Gelsinger DR, Wiegand T, Sternberg SH . Antagonistic conflict between transposon-encoded introns and guide RNAs. - bioRxiv: 2023 Nov 20; [PubMed:38045383]
[DOI]
- ↑ Nielsen H, Johansen SD . Group I introns: Moving in new directions. - RNA Biol: 2009 Sep-Oct, 6(4);375-83 [PubMed:19667762] [DOI]
- ↑ Hausner G, Hafez M, Edgell DR . Bacterial group I introns: mobile RNA catalysts. - Mob DNA: 2014 Mar 10, 5(1);8 [PubMed:24612670] [DOI]
- ↑ Tourasse NJ, Stabell FB, Kolstø AB . Survey of chimeric IStron elements in bacterial genomes: multiple molecular symbioses between group I intron ribozymes and DNA transposons. - Nucleic Acids Res: 2014 Nov 10, 42(20);12333-51 [PubMed:25324310] [DOI]
- ↑ Hasselmayer O, Braun V, Nitsche C, Moos M, Rupnik M, von Eichel-Streiber C . Clostridium difficile IStron CdISt1: discovery of a variant encoding two complete transposase-like proteins. - J Bacteriol: 2004 Apr, 186(8);2508-10 [PubMed:15060058] [DOI]
- ↑ Landthaler M, Shub DA . Unexpected abundance of self-splicing introns in the genome of bacteriophage Twort: introns in multiple genes, a single gene with three introns, and exon skipping by group I ribozymes. - Proc Natl Acad Sci U S A: 1999 Jun 8, 96(12);7005-10 [PubMed:10359829] [DOI]
- ↑ Golden BL, Kim H, Chase E . Crystal structure of a phage Twort group I ribozyme-product complex. - Nat Struct Mol Biol: 2005 Jan, 12(1);82-9 [PubMed:15580277] [DOI]
- ↑ Weiss A, Lopez CA, Beavers WN, Rodriguez J, Skaar EP . Clostridioides difficile strain-dependent and strain-independent adaptations to a microaerobic environment. - Microb Genom: 2021 Dec, 7(12); [PubMed:34908523] [DOI]
- ↑ Fuchs M, Lamm-Schmidt V, Sulzer J, Ponath F, Jenniches L, Kirk JA, Fagan RP, Barquist L, Vogel J, Faber F . An RNA-centric global view of Clostridioides difficile reveals broad activity of Hfq in a clinically important gram-positive bacterium. - Proc Natl Acad Sci U S A: 2021 Jun 22, 118(25); [PubMed:34131082] [DOI]
- ↑ 126.0 126.1 126.2
Chen W, Mandali S, Hancock SP, Kumar P, Collazo M, Cascio D, Johnson RC . Multiple serine transposase dimers assemble the transposon-end synaptic complex during IS607-family transposition. - Elife: 2018 Oct 5, 7; [PubMed:30289389]
[DOI]
- ↑ 127.0 127.1 127.2 127.3 Filée J, Siguier P, Chandler M . I am what I eat and I eat what I am: acquisition of bacterial genes by giant viruses. - Trends Genet: 2007 Jan, 23(1);10-5 [PubMed:17109990] [DOI]
- ↑ Filée J, Chandler M . Convergent mechanisms of genome evolution of large and giant DNA viruses. - Res Microbiol: 2008 Jun, 159(5);325-31 [PubMed:18572389] [DOI]
- ↑ Filée J, Chandler M . Gene exchange and the origin of giant viruses. - Intervirology: 2010, 53(5);354-61 [PubMed:20551687] [DOI]
- ↑ 130.0 130.1 130.2 130.3 130.4 130.5 130.6 Saito M, Xu P, Faure G, Maguire S, Kannan S, Altae-Tran H, Vo S, Desimone A, Macrae RK, Zhang F . Fanzor is a eukaryotic programmable RNA-guided endonuclease. - Nature: 2023 Aug, 620(7974);660-668 [PubMed:37380027] [DOI]
- ↑ Jiang K, Lim J, Sgrizzi S, Trinh M, Kayabolen A, Yutin N, Bao W, Kato K, Koonin EV, Gootenberg JS, Abudayyeh OO . Programmable RNA-guided DNA endonucleases are widespread in eukaryotes and their viruses. - Sci Adv: 2023 Sep 29, 9(39);eadk0171 [PubMed:37756409] [DOI]
- ↑ 132.0 132.1 Filée J, Pouget N, Chandler M . Phylogenetic evidence for extensive lateral acquisition of cellular genes by Nucleocytoplasmic large DNA viruses. - BMC Evol Biol: 2008 Nov 26, 8;320 [PubMed:19036122] [DOI]
- ↑ Volff JN . Turning junk into gold: domestication of transposable elements and the creation of new genes in eukaryotes. - Bioessays: 2006 Sep, 28(9);913-22 [PubMed:16937363] [DOI]
- ↑ Vogt A, Goldman AD, Mochizuki K, Landweber LF . Transposon domestication versus mutualism in ciliate genome rearrangements. - PLoS Genet: 2013, 9(8);e1003659 [PubMed:23935529] [DOI]
- ↑ Vogt A, Mochizuki K . A domesticated PiggyBac transposase interacts with heterochromatin and catalyzes reproducible DNA elimination in Tetrahymena. - PLoS Genet: 2013, 9(12);e1004032 [PubMed:24348275] [DOI]
- ↑ 136.0 136.1 136.2 Nunvar J, Huckova T, Licha I . Identification and characterization of repetitive extragenic palindromes (REP)-associated tyrosine transposases: implications for REP evolution and dynamics in bacterial genomes. - BMC Genomics: 2010 Jan 19, 11;44 [PubMed:20085626] [DOI]
- ↑ 137.0 137.1 137.2 Ton-Hoang B, Siguier P, Quentin Y, Onillon S, Marty B, Fichant G, Chandler M . Structuring the bacterial genome: Y1-transposases associated with REP-BIME sequences. - Nucleic Acids Res: 2012 Apr, 40(8);3596-609 [PubMed:22199259] [DOI]
- ↑ 138.0 138.1 Rocco F, De Gregorio E, Di Nocera PP . A giant family of short palindromic sequences in Stenotrophomonas maltophilia. - FEMS Microbiol Lett: 2010 Jul, 308(2);185-92 [PubMed:20528935] [DOI]
- ↑ 139.0 139.1 139.2 Nunvar J, Licha I, Schneider B . Evolution of REP diversity: a comparative study. - BMC Genomics: 2013 Jun 10, 14;385 [PubMed:23758774] [DOI]
- ↑ Gilson E, Bachellier S, Perrin S, Perrin D, Grimont PA, Grimont F, Hofnung M . Palindromic unit highly repetitive DNA sequences exhibit species specificity within Enterobacteriaceae. - Res Microbiol: 1990 Nov-Dec, 141(9);1103-16 [PubMed:2092362] [DOI]
- ↑ Boccard F, Prentki P . Specific interaction of IHF with RIBs, a class of bacterial repetitive DNA elements located at the 3' end of transcription units. - EMBO J: 1993 Dec 15, 12(13);5019-27 [PubMed:8262044] [DOI]
- ↑ Espéli O, Boccard F . In vivo cleavage of Escherichia coli BIME-2 repeats by DNA gyrase: genetic characterization of the target and identification of the cut site. - Mol Microbiol: 1997 Nov, 26(4);767-77 [PubMed:9427406] [DOI]
- ↑ Gilson E, Perrin D, Hofnung M . DNA polymerase I and a protein complex bind specifically to E. coli palindromic unit highly repetitive DNA: implications for bacterial chromosome organization. - Nucleic Acids Res: 1990 Jul 11, 18(13);3941-52 [PubMed:2197600] [DOI]
- ↑ Tobes R, Pareja E . Bacterial repetitive extragenic palindromic sequences are DNA targets for Insertion Sequence elements. - BMC Genomics: 2006 Mar 24, 7;62 [PubMed:16563168] [DOI]
- ↑ Liang W, Rudd KE, Deutscher MP . A role for REP sequences in regulating translation. - Mol Cell: 2015 May 7, 58(3);431-9 [PubMed:25891074] [DOI]
- ↑ Kofoid E, Bergthorsson U, Slechta ES, Roth JR . Formation of an F' plasmid by recombination between imperfectly repeated chromosomal Rep sequences: a closer look at an old friend (F'(128) pro lac). - J Bacteriol: 2003 Jan, 185(2);660-3 [PubMed:12511513] [DOI]
- ↑ 147.0 147.1 Bachellier S, Clément JM, Hofnung M . Short palindromic repetitive DNA elements in enterobacteria: a survey. - Res Microbiol: 1999 Nov-Dec, 150(9-10);627-39 [PubMed:10673002] [DOI]
- ↑ 148.0 148.1 Messing SA, Ton-Hoang B, Hickman AB, McCubbin AJ, Peaslee GF, Ghirlando R, Chandler M, Dyda F . The processing of repetitive extragenic palindromes: the structure of a repetitive extragenic palindrome bound to its associated nuclease. - Nucleic Acids Res: 2012 Oct, 40(19);9964-79 [PubMed:22885300] [DOI]
- ↑ 149.0 149.1 Beuzón CR, Chessa D, Casadesús J . IS200: an old and still bacterial transposon. - Int Microbiol: 2004 Mar, 7(1);3-12 [PubMed:15179601]
- ↑ 150.00 150.01 150.02 150.03 150.04 150.05 150.06 150.07 150.08 150.09 150.10 150.11 Ellis MJ, Trussler RS, Charles O, Haniford DB . A transposon-derived small RNA regulates gene expression in Salmonella Typhimurium. - Nucleic Acids Res: 2017 May 19, 45(9);5470-5486 [PubMed:28335027] [DOI]
- ↑ Casadesus J, Roth JR . Transcriptional occlusion of transposon targets. - Mol Gen Genet: 1989 Apr, 216(2-3);204-9 [PubMed:2546037] [DOI]
- ↑ Schiaffino A, Beuzón CR, Uzzau S, Leori G, Cappuccinelli P, Casadesús J, Rubino S . Strain typing with IS200 fingerprints in Salmonella abortusovis. - Appl Environ Microbiol: 1996 Jul, 62(7);2375-80 [PubMed:8779575] [DOI]
- ↑ 153.0 153.1 153.2 153.3 153.4 153.5 Ellis MJ, Carfrae LA, Macnair CR, Trussler RS, Brown ED, Haniford DB . Silent but deadly: IS200 promotes pathogenicity in Salmonella Typhimurium. - RNA Biol: 2018 Feb 1, 15(2);176-181 [PubMed:29120256] [DOI]
- ↑ 154.0 154.1 154.2 154.3 154.4 154.5 154.6 Ellis MJ, Trussler RS, Haniford DB . A cis-encoded sRNA, Hfq and mRNA secondary structure act independently to suppress IS200 transposition. - Nucleic Acids Res: 2015 Jul 27, 43(13);6511-27 [PubMed:26044710] [DOI]
- ↑ Ross JA, Ellis MJ, Hossain S, Haniford DB . Hfq restructures RNA-IN and RNA-OUT and facilitates antisense pairing in the Tn10/IS10 system. - RNA: 2013 May, 19(5);670-84 [PubMed:23510801] [DOI]
- ↑ 156.0 156.1 Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, Hinton JC, Vogel J . Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. - PLoS Genet: 2008 Aug 22, 4(8);e1000163 [PubMed:18725932] [DOI]
- ↑ Kröger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, Chao Y, Sittka A, Hébrard M, Händler K, Colgan A, Leekitcharoenphon P, Langridge GC, Lohan AJ, Loftus B, Lucchini S, Ussery DW, Dorman CJ, Thomson NR, Vogel J, Hinton JC . The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. - Proc Natl Acad Sci U S A: 2012 May 15, 109(20);E1277-86 [PubMed:22538806] [DOI]
- ↑ Yan Y, Su S, Meng X, Ji X, Qu Y, Liu Z, Wang X, Cui Y, Deng Z, Zhou D, Jiang W, Yang R, Han Y . Determination of sRNA expressions by RNA-seq in Yersinia pestis grown in vitro and during infection. - PLoS One: 2013, 8(9);e74495 [PubMed:24040259] [DOI]
- ↑ Hershko-Shalev T, Odenheimer-Bergman A, Elgrably-Weiss M, Ben-Zvi T, Govindarajan S, Seri H, Papenfort K, Vogel J, Altuvia S . Gifsy-1 Prophage IsrK with Dual Function as Small and Messenger RNA Modulates Vital Bacterial Machineries. - PLoS Genet: 2016 Apr, 12(4);e1005975 [PubMed:27057757] [DOI]
- ↑ Chao Y, Vogel J . A 3' UTR-Derived Small RNA Provides the Regulatory Noncoding Arm of the Inner Membrane Stress Response. - Mol Cell: 2016 Feb 4, 61(3);352-363 [PubMed:26805574] [DOI]
- ↑ Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J . An atlas of Hfq-bound transcripts reveals 3' UTRs as a genomic reservoir of regulatory small RNAs. - EMBO J: 2012 Oct 17, 31(20);4005-19 [PubMed:22922465] [DOI]
- ↑ Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G . MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. - Genes Dev: 2014 Jul 15, 28(14);1620-34 [PubMed:25030700] [DOI]
- ↑ Jørgensen MG, Thomason MK, Havelund J, Valentin-Hansen P, Storz G . Dual function of the McaS small RNA in controlling biofilm formation. - Genes Dev: 2013 May 15, 27(10);1132-45 [PubMed:23666921] [DOI]
- ↑ Holmqvist E, Wright PR, Li L, Bischler T, Barquist L, Reinhardt R, Backofen R, Vogel J . Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. - EMBO J: 2016 May 2, 35(9);991-1011 [PubMed:27044921] [DOI]
- ↑ van Nues RW, Castro-Roa D, Yuzenkova Y, Zenkin N . Ribonucleoprotein particles of bacterial small non-coding RNA IsrA (IS61 or McaS) and its interaction with RNA polymerase core may link transcription to mRNA fate. - Nucleic Acids Res: 2016 Apr 7, 44(6);2577-92 [PubMed:26609136] [DOI]
- ↑ Ellis MJ, Trussler RS, Haniford DB . Hfq binds directly to the ribosome-binding site of IS10 transposase mRNA to inhibit translation. - Mol Microbiol: 2015 May, 96(3);633-50 [PubMed:25649688] [DOI]
- ↑ Jäger D, Pernitzsch SR, Richter AS, Backofen R, Sharma CM, Schmitz RA . An archaeal sRNA targeting cis- and trans-encoded mRNAs via two distinct domains. - Nucleic Acids Res: 2012 Nov, 40(21);10964-79 [PubMed:22965121] [DOI]
- ↑ Vogel J, Luisi BF . Hfq and its constellation of RNA. - Nat Rev Microbiol: 2011 Aug 15, 9(8);578-89 [PubMed:21760622] [DOI]
- ↑ Myeni SK, Wang L, Zhou D . SipB-SipC complex is essential for translocon formation. - PLoS One: 2013, 8(3);e60499 [PubMed:23544147] [DOI]
- ↑ 170.0 170.1 170.2 A small RNA derived from the 5’ end of the IS200 tnpA transcript regulates multiple virulence regulons in Salmonella Typhimurium. Ryan S. Trussler, Naomi-Jean Q. Scherba, Michael J. Ellis, Konrad U. Förstner, Matthew Albert, Alexander J. Westermann, David B. Haniford. bioRxiv 2024.06.26.600842; doi: https://doi.org/10.1101/2024.06.26.600842
- ↑ 171.0 171.1 Lou L, Zhang P, Piao R, Wang Y . Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. - Front Cell Infect Microbiol: 2019, 9;270 [PubMed:31428589] [DOI]
- ↑ Huang CJ, Adler BA, Doudna JA . A naturally DNase-free CRISPR-Cas12c enzyme silences gene expression. - Mol Cell: 2022 Jun 2, 82(11);2148-2160.e4 [PubMed:35659325] [DOI]
- ↑ Wu WY, Mohanraju P, Liao C, Adiego-Pérez B, Creutzburg SCA, Makarova KS, Keessen K, Lindeboom TA, Khan TS, Prinsen S, Joosten R, Yan WX, Migur A, Laffeber C, Scott DA, Lebbink JHG, Koonin EV, Beisel CL, van der Oost J . The miniature CRISPR-Cas12m effector binds DNA to block transcription. - Mol Cell: 2022 Dec 1, 82(23);4487-4502.e7 [PubMed:36427491] [DOI]
- ↑ 174.00 174.01 174.02 174.03 174.04 174.05 174.06 174.07 174.08 174.09 174.10 174.11 174.12 174.13 174.14 174.15 Wiegand T, Hoffmann FT, Walker MWG, Tang S, Richard E, Le HC, Meers C, Sternberg SH . Emergence of RNA-guided transcription factors via domestication of transposon-encoded TnpB nucleases. - bioRxiv: 2023 Nov 30; [PubMed:38076855] [DOI]
- ↑ 175.00 175.01 175.02 175.03 175.04 175.05 175.06 175.07 175.08 175.09 175.10 175.11 175.12 175.13 175.14 175.15 Wiegand T, Hoffmann FT, Walker MWG, Tang S, Richard E, Le HC, Meers C, Sternberg SH . TnpB homologues exapted from transposons are RNA-guided transcription factors. - Nature: 2024 Jul, 631(8020);439-448 [PubMed:38926585] [DOI]
- ↑ Siguier P, Gourbeyre E, Chandler M . Bacterial insertion sequences: their genomic impact and diversity. - FEMS Microbiol Rev: 2014 Sep, 38(5);865-91 [PubMed:24499397] [DOI]
- ↑ 177.0 177.1 He S, Corneloup A, Guynet C, Lavatine L, Caumont-Sarcos A, Siguier P, Marty B, Dyda F, Chandler M, Ton Hoang B . The IS200/IS605 Family and "Peel and Paste" Single-strand Transposition Mechanism. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350330] [DOI]
- ↑ Jiang K, Lim J, Sgrizzi S, Trinh M, Kayabolen A, Yutin N, Bao W, Kato K, Koonin EV, Gootenberg JS, Abudayyeh OO . Programmable RNA-guided DNA endonucleases are widespread in eukaryotes and their viruses. - Sci Adv: 2023 Sep 29, 9(39);eadk0171 [PubMed:37756409] [DOI]
- ↑ 179.0 179.1 179.2 Meers C, Le HC, Pesari SR, Hoffmann FT, Walker MWG, Gezelle J, Tang S, Sternberg SH . Transposon-encoded nucleases use guide RNAs to promote their selfish spread. - Nature: 2023 Oct, 622(7984);863-871 [PubMed:37758954] [DOI]
- ↑ 180.0 180.1 Michaux C, Hansen EE, Jenniches L, Gerovac M, Barquist L, Vogel J . Single-Nucleotide RNA Maps for the Two Major Nosocomial Pathogens Enterococcus faecalis and Enterococcus faecium. - Front Cell Infect Microbiol: 2020, 10;600325 [PubMed:33324581] [DOI]
- ↑ Nety SP, Altae-Tran H, Kannan S, Demircioglu FE, Faure G, Hirano S, Mears K, Zhang Y, Macrae RK, Zhang F . The Transposon-Encoded Protein TnpB Processes Its Own mRNA into ωRNA for Guided Nuclease Activity. - CRISPR J: 2023 Jun, 6(3);232-242 [PubMed:37272862] [DOI]
- ↑ Swarts DC, van der Oost J, Jinek M . Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. - Mol Cell: 2017 Apr 20, 66(2);221-233.e4 [PubMed:28431230] [DOI]